Antibody-Drug Conjugates (ADCs) represent a novel drug modality designed for cell-specific delivery of therapeutic payloads, with monoclonal antibodies (mAbs) serving as the guiding mechanism.
Image Credit: BioIVT
The precise delivery of the payload relies on the specificity of the mAb and the unique presentation of the selected epitope. Ideally, the chosen epitope is present exclusively and abundantly on diseased cells.
Typically, the therapeutic molecule, known as the ADC payload, is a highly potent small-molecule cytotoxin that delivers the therapeutic effect. A linker connects the payload to the mAb, ensuring stability, non-interference with mAb binding, and controlled release of the payload once it reaches its target.
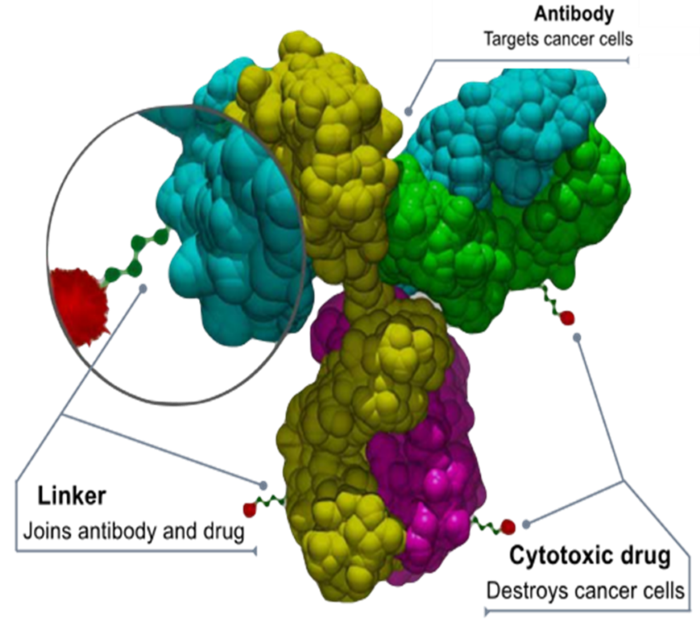
Image Source: https://commons.wikimedia.org/wiki/File:Antibody-drug_conjugate_structure.svg
ADCs approved for clinical use
Source: BioIVT
| ADC, year approved |
Indication |
Target, payload (mechanism) |
Boxed warning |
| Gemtuzumab ozogamicin, 2000, 2017 |
Acute myeloid leukemia |
CD33, calicheamicin (DNA cleavage) |
Hepatic veno-occlusive disease, also known as sinusoidal obstruction syndrome |
| Brentuximab vedotin, 2011 |
Hodgkin’s lymphoma, anaplastic large cell lymphoma |
CD30, auristatin E (microtubule inhibitor) |
Progressive multifocal leukoencephalopathy |
| Trastuzumab emtansine, 2013 |
HER-2+ breast cancer (after prior anti-HER-2 therapy) |
HER-2, maytansine (microtubule inhibitor |
Hepatotoxicity, cardiac toxicity, embryo-fetal toxicity |
| lnotuzumab ozogamicin, 2017 |
B-cell precursor acute lymphoblastic leukemia |
CD22, calicheamicin (DNA cleavage) |
Hepatic veno-occlusive disease, also known as sinusoidal obstruction syndrome |
| Moxetumomab pasudotox, 2018 |
Hairy cell leukemia |
CD22, pseudomonas exotoxin A (apoptosis) |
Capillary leak syndrome, hemolytic uremic syndrome |
| Polatuzumab vedotin, 2019 |
Diffuse large B-cell lymphoma |
CD79b, auristatin E (microtubule inhibitor) |
NA |
| Enfortumab vedotin, 2019 |
Urothelial cancer |
Nectin-4, auristatin E (microtubule inhibitor) |
NA |
| Trastuzumab deruxtecan, 2019 |
HER-2+ breast cancer, unresectable or metastatic non-small cell lung cancer |
HER-2, deruxtecan (topoisomerase inhibitor) |
Interstitial lung disease, embryo-fetal toxicity |
| Sacituzumab govitecan, 2020 |
Metastatic triple-negative breast cancer |
Trop-2, SN-38 (a topoisomerase inhibitor) |
Neutropenia, diarrhea |
| Tisotumab vedotin, 2021 |
Metastatic cervical cancer |
FT, auristatin E (microtubule inhibitor) |
Ocular toxicity |
| Loncastuximab tesirine, 2021 |
Large B-cell lymphoma |
CD19, pyrrolobenzodiazepine dimer (DNA crosslinking) |
NA |
| Mirvetuximab soravtansine, 2022 |
Platinum-resistant ovarian cancer |
FRα, maytansinoid DM4 (microtubule inhibitor) |
Ocular toxicity |
The table above shows the twelve FDA-approved ADC currently available on the market. Other ADC include:
- Belantamab mafodotin was approved in 2020 for treating multiple myeloma but later withdrawn in 2022 due to its lack of efficacy. This ADC carried auristatin F.
- Cetuximab sarotalocan, which is an antibody-photoabsorber conjugate developed and approved in Japan.
- Disitamab vedotin, which was developed and approved in China.
Source: BioIVT
| ADC, year approved |
Indication |
Target, payload (mechanism) |
| Cetuximab sarotalocan, 2020 |
Head and neck cancers |
EGFR, IR700 (photosensitizer) |
| Disitamab vedotin, 2021 |
HER-2 positive gastric cancer and breast cancer |
HER-2, auristatin E (microtubule inhibitor) |
ADC indications and targets
ADC indications include:
- Hematological malignancies such as Hodgkin's lymphoma, Leukemia, and anaplastic large cell lymphoma.
- Solid tumors such as HER-2+ breast cancer, non-small cell lung cancer, urothelial, cervical and ovarian cancer, and locally advanced or metastatic HER-2+ gastric or gastroesophageal junction adenocarcinoma.
ADC targets include epitopes expressed uniquely on cancer cells. These are typically—but not always—ideal targets to limit the ADC's off-target toxicity.
Multiple ADCs can also be used to target selective markers. For example, HER-2 can be targeted using trastuzumab emtansine, a topoisomerase inhibitor, and a microtubule inhibitor, as well as trastuzumab deruxtecan. Similarly, CD22 can be targeted using inotuzumab ozogamicin, which induces DNA cleavage, or moxetumomab pasudotox, which leads to apoptosis.
Payloads: The mechanism of therapeutic action
All current payloads are derivatives of natural products that are too toxic for application without a guiding mechanism.
Repeated evaluation of certain drug-drug interaction (DDI) properties of established payloads, such as auristatin, could be replaced by modeling. For instance, pseudomonas exotoxin A, the payload in moxetumomab pasudotox, did not undergo evaluation for DDI perpetrator potential, as peptides are generally not expected to induce or inhibit enzymes or transporters.
Mechanism of action
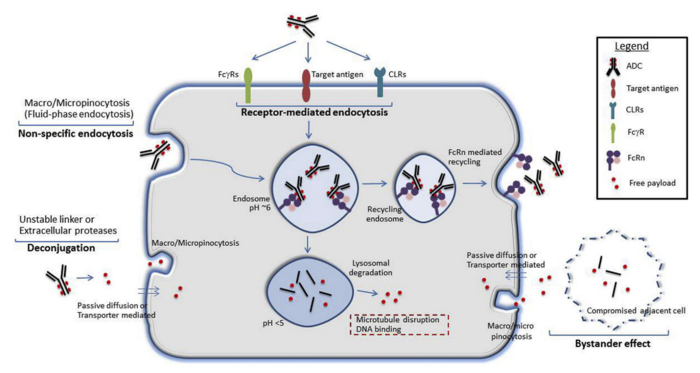
Image Credit: P.K. Mahalingaiah et al., Pharmacology & Therapeutics 200 (2019) 110–125
Receptor-mediated endocytosis is the intended mechanism of ADC entry into target cells, occurring after the conjugate binds to the antigen on the cell surface.
Other receptors, such as FcɣRs or CLRs, can facilitate non-specific uptake of the ADC, providing an additional route of entry into the tumor cell. Deconjugated payloads may also enter the cell through pinocytosis, passive diffusion, or transporter-mediated uptake.
Once inside the cell, endocytosis directs the ADC into endosomes. However, it is important to note that some endosomes enable FcRn-mediated recycling of immunoglobulin G, potentially diverting part of the ADC dose out of the cell before payload release.
Another key mechanism for payload release involves proteolytic degradation within lysosomes, which leads to the distribution of the payload across cell compartments, delivering its therapeutic effects.
A process known as the 'bystander effect' can occur when the unconjugated payload is released into the tumor microenvironment via pinocytosis, potentially compromising adjacent healthy cells.
Box warnings: Toxicities
Source: BioIVT
| ADC |
Indication, payload (mechanism) |
Boxed warning |
| Gemtuzumab ozogamicin |
Acute myeloid leukemia, calicheamicin (DNA cleavage) |
Hepatic veno-occlusive disease, also known as sinusoidal obstruction syndrome |
| Brentuximab vedotin |
Hodgkin lymphoma, anaplastic large cell lymphoma, auristatin (microtubule inhibitor) |
Progressive multifocal leukoencephalopathy |
| Trastuzumab emtansine |
HER-2+ breast cancer, maytansine (microtubule inhibitor) |
Hepatotoxicity, cardiac toxicity, embryo-fetal toxicity |
| lnotuzumab ozogamicin |
B-cell precursor acute lymphoblastic leukemia, calicheamicin (DNA cleavage) |
Hepatic veno-occlusive disease, also known as sinusoidal obstruction syndrome |
| Moxetumomab pasudotox |
Hairy cell leukemia, pseudomonas exotoxin A (induction of apoptosis) |
Capillary leak syndrome, hemolytic uremic syndrome |
| Trastuzumab deruxtecan |
HER-2+ breast cancer, non-small cell lung cancer, deruxtecan (topoisomerase inhibitor) |
Interstitial lung disease, embryo-fetal toxicity |
| Sacituzumab govitecan |
Metastatic triple-negative breast cancer, SN-38 (topoisomerase inhibitor) |
Neutropenia, diarrhea |
| Tisotumab vedotin |
Metastatic cervical cancer, auristatin (microtubule inhibitor) |
Ocular toxicity |
| Mirvetuximab soravtansine |
Platinum-resistant ovarian cancer maytansinoid DM4 (microtubule inhibitor) |
Ocular toxicity |
Boxed warnings, assigned to 75 % of FDA-approved ADCs, highlight the serious toxicities associated with these drugs. The complexity of ADCs, including the properties of payloads, monoclonal antibodies (mAbs), linkers, and the underlying disease processes, may all contribute to the observed toxicities.
Among the four auristatin-containing ADCs, only one carries a boxed warning, suggesting that the payload is just one of several factors contributing to the overall toxicity.
Ocular toxicity
The risk of ocular toxicity is documented in box warnings for tisotumab vedotin and mirvetuximab soravtansine.
The interactions between the payload and the linker are considered key factors associated with this ADC-related toxicity.
In the case of cleavable linkers, early release of the free payload into circulation can lead to cytotoxicity, while non-cleavable linkers may contribute to toxicity and its related side effects via prolonged circulation of the intact ADC and late release in variable locations, such as the eyes (2023 ESMO annual meeting).
Source: BioIVT
| |
|
|
Tisotumab vedotin
Metastatic cervical cancer
Tissue factor |
Auristatin E (microtubule inhibitor) |
Cleavable linker |
Mirvetuximab soravtansine
Platinum-resistant ovarian cancer
Folate receptor alpha |
Maytansinoid DM4 (microtubule inhibitor) |
Cleavable linker |
Toxicity of sacituzumab govitecan payload SN-38
SN-38 is an active metabolite of the cancer drug irinotecan. This particular ADC has been approved for treating breast and urothelial metastatic cancers, though it does feature a warning that is an extension of irinotecan’s box warnings.
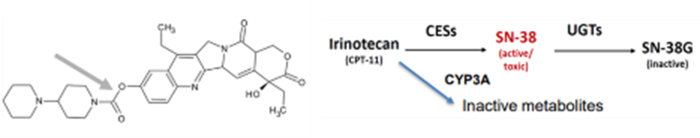
Image Credit: BioIVT
Irinotecan is metabolized into inactive compounds by cytochrome P450 (CYP) 3A4 and activated by carboxylesterases (CES) into SN-38. Hepatic UDP-glucuronosyltransferases (UGT) 1A1 and UGT1A9 then inactivate SN-38.
Patients with the UGT1A1*28/*28 genotype are at higher risk for neutropenia, febrile neutropenia, and anemia. Concomitant use of sacituzumab govitecan (Trodelvy) with UGT1A1 inhibitors or inducers should be avoided, as indicated in the drug label.
Toxicities of ADC targeting TROP-2, NECTIN-4, and HER-2 in urothelial carcinoma
ADC targeting TROP-2, NECTIN-4, and HER-2 (sacituzumab govitecan, enfortumab vedotin, and disitamab vedotin) are highly effective in terms of their intra-tumoral drug concentration and their capacity to kill cancer cells. They also perform well in terms of reducing systemic distribution and limiting off-target effects.
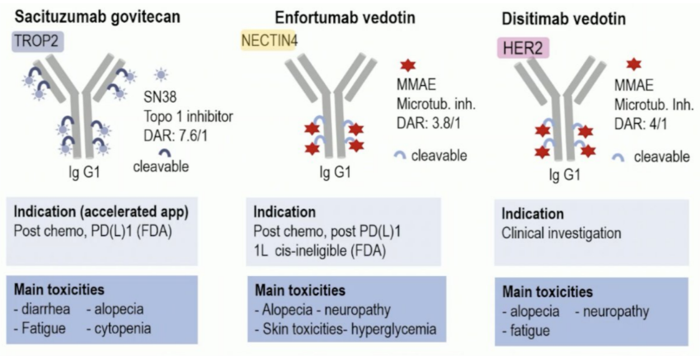
Image Credit: 2023 ESMO annual meeting, Dr. Yohann Loriot, UroToday.com
The main toxicities of these three ADCs differ. Cytopenia is observed with sacituzumab govitecan (SN-38 topoisomerase inhibitor) and is a major toxicity of irinotecan, whereas skin toxicities and hyperglycemia distinguish the two auristatin-based (microtubule inhibitor) ADCs, which have comparable drug-to-antibody ratios (DAR).
The specificity of enfortumab or the distribution of NECTIN-4 contributes to these particular toxicities. NECTIN-4 expression is approximately four times higher in normal skin than in the urinary bladder, which may explain why skin toxicities are seen with enfortumab vedotin but not with disitamab vedotin in urothelial carcinoma patients. This expression ratio is expected to decrease in cancerous tissues.
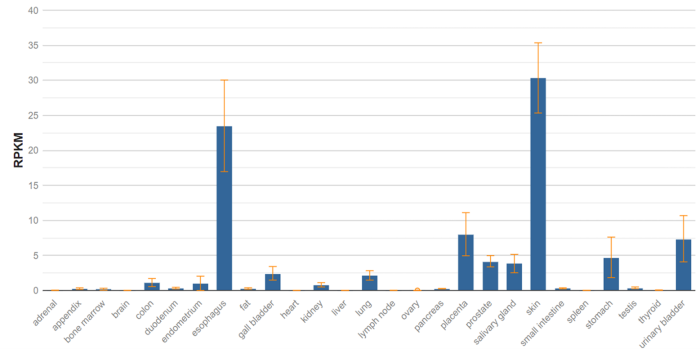
Image Credit: National Library of Medicine, Gene ID: 81607
Metabolism-mediated reactions of brentuximab vedotin
Brentuximab vedotin gained regulatory approval in 2011. While drug-drug interactions involving antibodies are generally limited, it is possible for the cytotoxic agent portion of an ADC to be subject to metabolism-based DDIs.
For example, monomethyl auristatin E (MMAE)—a brentuximab vedotin payload—is a substrate of CYP3A and P-glycoprotein (P-gp). This means that its conjugate exhibits victim potential and is also a potential inhibitor of CYP3A but no other CYP isoforms (J Clin Pharmacol. 2013 August; 53(8): 866–877).
During the clinical study, brentuximab vedotin exhibited a number of notable characteristics.
- It did not impact the pharmacokinetics (PK) of midazolam, a sensitive CYP3A4 substrate.
- Rifampin, an inducer of CYP3A4, was found to decrease plasma exposure of MMAE.
- Ketoconazole, a CYP3A4 inhibitor, was found to increase plasma exposure of MMAE.
At least a portion of MMAE measured in plasma likely entered the cytoplasm of tumor cells or hepatocytes, where it was either metabolized or exited the cells intact through passive diffusion or transporter-mediated efflux.
The study did not analyze potential target or off-target toxic effects of CYP3A4 induction or inhibition. According to brentuximab vedotin's label, concomitant use of strong CYP3A4 inhibitors, inducers, or P-gp inhibitors may affect MMAE exposure.
Regulatory guidance for safety evaluation of ADC-ICH Drug Interaction Studies M12 Guideline
For ADCs, the small molecule drug component conjugated to the antibody can be released in its unconjugated form. Regulatory guidance suggests that the DDI potential of both the antibody and the small molecule drug component should be evaluated.
However, in many cases, the systemic concentration of the free drug may be too low to act as a perpetrator in vivo.
A clinical study of brentuximab vedotin highlights this concern, where the payload's perpetrator potential was evaluated, and it was found that the concentration of free auristatin E was too low to have a clinically significant effect on the PK of midazolam.
The study in question does not discuss the DDI potential of the intact ADC or the antibody itself, although it is anticipated that this would be low. The antibody is catabolized to small peptides and individual amino acids, meaning that it does not exhibit the potential to induce or inhibit enzymes or transporters.
Understanding the formation, distribution, and elimination kinetics of the small molecule payload is crucial, as is assessing its systemic exposure. If increased levels of free drug are associated with safety concerns, it may be necessary to evaluate the small molecule component as a victim of drug interactions.
For example, sacituzumab govitecan carries the SN-38 payload, a metabolite of irinotecan that is deactivated by hepatic UGT1A1 and UGT1A9. UGT1A1, in particular, is polymorphically expressed, and variations in its activity can influence toxicity.
Understanding the formation, distribution, and elimination kinetics of the small molecule payload is crucial, as is assessing its systemic exposure. If increased levels of free drug are associated with safety concerns, it may be necessary to evaluate the small molecule component as a victim of drug interactions.
For example, sacituzumab govitecan carries the SN-38 payload, a metabolite of irinotecan that is deactivated by hepatic UGT1A1 and UGT1A9. UGT1A1, in particular, is polymorphically expressed, and variations in its activity can influence toxicity.
The DDI potential of the ADC may be related to the payload being release from the ADC before reaching its target or being released extracellularly from the cancer cell after it has been killed. Stability of the ADC in plasma and the concentration of the payload in plasma must be evaluated in vitro and in vivo.
Strategies for ADME characterization of ADC payloads and in vitro studies of drug interaction potential of ADC
BioIVT’s comprehensive characterization strategy for small molecule ADC payloads comprises a number of steps.
- Identification of major ADC metabolites in plasma, lysosomes, and hepatic or tumor subcellular fractions
- Assessment of major metabolites as a victim of drug interactions (via reaction phenotyping or transporter substrate potential), and as a perpetrator (via enzyme induction and enzyme and transporter inhibition)
- Thorough assessment of in vitro data in line with regulatory guidance and recommendations
The table below provides information on a range of major studies conducted by BioIVT to evaluate the drug interaction potential of small molecule ADC payloads.
Source: BioIVT
| Metabolism-mediated interactions |
Test system |
| Is the payload a substrate of drug-metabolizing enzymes? Conduct a metabolic stability study and reaction phenotyping studies. |
Hepatocytes, microsomes, recombinant enzymes |
| Is the payload an inhibitor of drug-metabolizing enzymes? Evaluate direct and metabolism-dependent inhibition of CYP and potentially UGT enzymes. |
Microsomes, hepatocytes |
| Is the payload an inducer of drug-metabolizing enzymes? Conduct a CYP induction study in hepatocytes from three donors. |
Cultured hepatocytes |
| Transporter-mediated interactions |
Test system |
Is the payload a substrate of drug transporters?
Conduct a drug transporters substrate potential study. |
Cell lines, membrane vesicles |
Is the payload an inhibitor of drug transporters?
Conduct a drug transporters inhibitor potential study. |
Cell lines, membrane vesicles |
| Metabolite-mediated interactions |
Test system |
| Questions analogous to the parent molecule may need to be answered for metabolites of the payload. |
Multiple systems |
Conclusion
Antibody-drug conjugates represent an extremely promising modality for meeting the needs of patients with significant health issues. However, the highly cytotoxic payloads employed in ADC for oncology present specific challenges in terms of the development of safe and effective molecules.
Numerous mechanisms, including individual conjugate components, disease state, and patient characteristics, are likely to contribute to the observed toxicities of the ADC.
Understanding these potential risks allows for the safer use of these life-saving therapeutics, and in some cases, some of the critical properties of the ADC can be improved based on in vitro studies.
Acknowledgments
Produced from materials originally authored by BioIVT.
About BioIVT
BioIVT, formerly BioreclamationIVT, is a leading global provider of high-quality biological specimens and value-added services. We specialize in control and disease state samples including human and animal tissues, cell products, blood and other biofluids. Our unmatched portfolio of clinical specimens directly supports precision medicine research and the effort to improve patient outcomes by coupling comprehensive clinical data with donor samples.
Our Research Services team works collaboratively with clients to provide in vitro hepatic modeling solutions. And as the world’s premier supplier of ADME-Tox model systems, including hepatocytes and subcellular fractions, BioIVT enables scientists to better understand the pharmacokinetics and drug metabolism of newly discovered compounds and the effects on disease processes. By combining our technical expertise, exceptional customer service, and unparalleled access to biological specimens, BioIVT serves the research community as a trusted partner in ELEVATING SCIENCE®.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.