For pharmaceutical and CDMO testing laboratories subject to stringent regulation, direct, real-time mass spectrometry has the potential to reduce analytical process times significantly due to minimal sample preparation, rapid sample analysis, and the flexibility column-free techniques afford.
However, the pharmaceutical industry has still not taken full advantage of real-time mass spectrometry due to a lack of instrumentation designed to closely follow FDA 21 CFR Part 11 compliant analyses and processes.

Image Credit: IM Imagery/Shutterstock.com
Syft Tracer Pharm11 is the first real-time mass spectrometry solution developed specifically with 21 CFR Part 11 in mind. This solution offers several benefits, including full, high-throughput mass spectrometry for pharmaceutical and CDMO workflows in combination with direct, real-time selected ion flow tube mass spectrometry (SIFT-MS) analysis.1,2
Users of the Syft Tracer Pharm11 will benefit from the ability to conduct measurements of multiple, diverse compounds in a single configuration without requiring physical changeover routines between methods. Additionally, the system facilitates >200 samples per day throughput, and users are not impeded by time-consuming, complex chromatographic separation.
The Syft Tracer Pharm11 is comprised of the next-generation SIFT-MS Syft Tracer platform, integrated CTC autosampler, high-throughput inlet, and the SyftAuditTracer software package.
SyftAuditTracer allows laboratories to achieve 21 CFR Part 11 compliance. It enables complete traceability through data acquisition and processing, plus comprehensive user management functionality.
This article outlines how this industry-first product has been developed to meet compliance with 21 CFR Part 11 by presenting an analytical workflow in which important regulatory compliance features are highlighted.
Introduction
Investigating impurity levels in pharmaceutical drug substances, excipients, and products can run up costs as it requires highly skilled analysts and specialist instrumentation. Resultingly, a laboratory may experience costly periods of downtime if performing tens or even hundreds of different techniques, as instrumentation must be recalibrated between assays.
Even if a validated method exists, results acquisition can still take days from receipt of samples; this is due to complex sample preparation procedures and prolonged analytical run times. Any kind of delay can be problematic in manufacturing environments when time to data is a key factor.
Real-time, direct mass spectrometry overcomes many of the issues associated with conventional analytical techniques. Due to its flexibility, absence of chromatography, and high-throughput capability, it offers significant time and cost savings.
SIFT-MS is a well-established direct mass spectrometry technique for the real-time analysis of volatile compounds. SIFT-MS instruments, such as the Syft Tracer, have been specifically designed to quantify VOCs and other small, volatile species (mass range 15-400 Da) down to part-per-trillion concentrations (pptV in the gas phase).3
These specialist instruments deliver rapid results, column-free selectivity through different reagent ions, and a universal configuration, eliminating the need to reconfigure the system between analytical methods.3,4,5
Syft Tracer Pharm11 was specifically developed as an analytical solution for the pharmaceutical industry. In Syft Tracer Pharm11, the Syft Tracer base platform offers native time efficiency, which is optimized by the autosampler integration and sample inlet, aimed at delivering high-throughput analysis.
The system incorporates SyftAuditTracer software, providing analytical solutions through complete 21 CFR Part 11 support for the instrumentation while facilitating simple, fast, automated workflows.
However, despite the benefits SIFT-MS offers, adoption of the technique by the pharmaceutical industry has been impeded by the need to comply with the requirements of the FDA’s 21 CFR Part 11 regulations.
Previously, there has been a lack of real-time mass spectrometry data acquisition and processing software specifically designed to meet 21 CFR Part 11 compliance. With this gap in the market, by incorporating the unique characteristics of SIFT-MS, a new solution for regulated environments is now available.
The Syft Tracer Pharm11 is a complete package solution for 21 CFR Part 11 compliant workflows. This article highlights the 21 CFR Part 11-related features that are incorporated into the SyftAuditTracer by detailing an example workflow. Moreover, technical controls included in SyftAuditTracer are shown in the appendix compared against the relevant 21 CFR Part 11 regulation.
Executing a workflow in a regulated laboratory
This section provides a step-by-step guide on conducting an analysis using the Syft Tracer Pharm11 with the SyftAuditTracer software, from setting up the instrument for data collection to delivering analytical findings. It also highlights how the SyftAuditTracer software is built to adhere to the regulations specified in 21 CFR Part 11.
This workflow example will be from the perspective of a user with analyst experience, which allows them to perform method creation, data acquisition, data analysis, and reporting tasks. The objective is to outline the workflow clearly, avoiding detailed technical discussions.
Logging into SyftAuditTracer
Users must log in to SyftAuditTracer to enter the compliant environment. To ensure secure access to the SyftAuditTracer, the system administrator provides login credentials that are unique to each user.
At each login, the audit trail within the software records the relevant time, date, and user ID. Any actions performed are attributed to the associated user until logout; this ensures accountability and traceability.
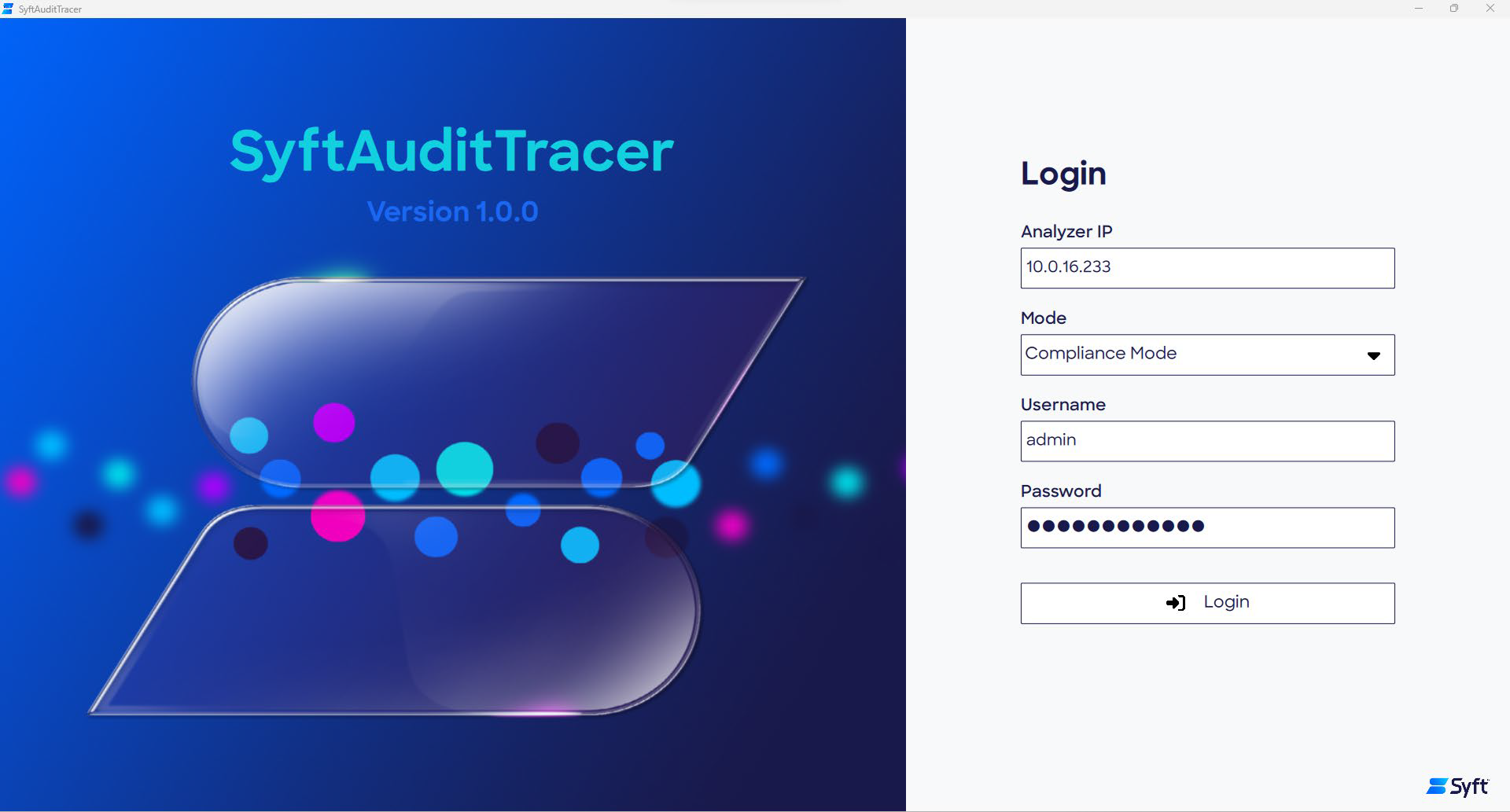
Figure 1. SyftAuditTracer login page. Image Credit: Syft Technologies
Method creation
Prior to sample analysis, it may be necessary to create or amend analytical methods within SyftAuditTracer. Two major method types are used: SIFT-MS methods and autosampler methods.
SIFT-MS methods are generated externally in the LabSyft software and subsequently transferred to SyftAuditTracer, where they can be managed in line with 21 CFR Part 11.
It is possible to create and manage autosampler method files for headspace analysis directly within SyftAuditTracer. When each method has been finalized and saved in the system, they become non-editable files upon saving and are stored in the compliant database.
To create new files, the existing method files can be used as a template, however, each new edit must always be saved under a new unique file name in SyftAuditTracer. To preserve close control of the validity of methods, SyftAuditTracer imposes an approval process for SIFT-MS and autosampler methods.
Before these methods can be employed in 21 CFR Part 11 compliant sample analyses, they must receive approval from a user with the appropriate permissions. Method creation, approval, and sequencing permissions are set and can be customized by the Administrator on the Profiles page.
SyftAuditTracer incorporates robust measures to protect the integrity of method files:
- Protection from loss: The risk of data loss is mitigated by preventing the editing or deletion of methods saved in SyftAuditTracer.
- Unique file IDs: To prevent duplication of methods, each method file must have a unique file ID.
- Cryptographic hashing: Method file integrity verification is ensured by applying a checksum to each file.
- Audit trail: Traceability and accountability through user ID capture when creating and approving method files, which logs user IDs, date, and time information. Any edits require saving under a new file ID, ensuring a complete version history of all method files.
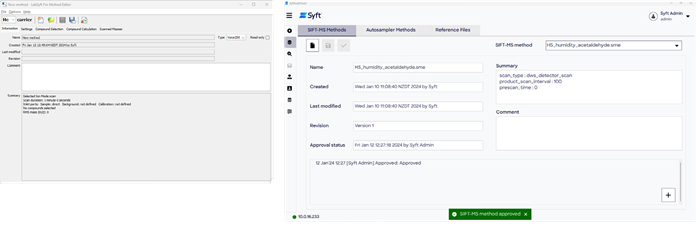
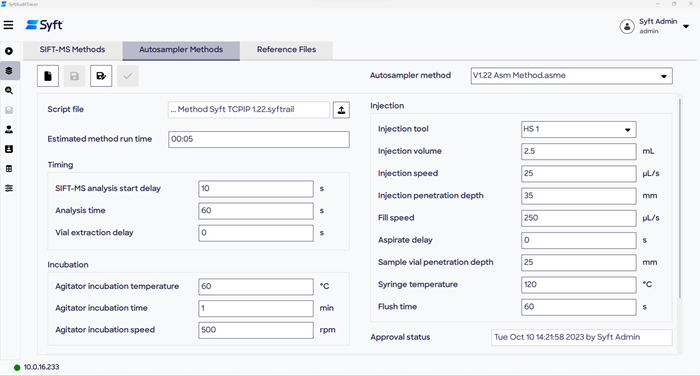
Figure 2. Clockwise from top left: the Method Editor from the LabSyft software suite, the SIFT-MS Methods page in SyftAuditTracer, and the Autosampler Methods page in SyftAuditTracer. Image Credit: Syft Technologies
Preparing for analysis
The instrument’s status can be closely monitored via the Run page of SyftAuditTracer, which enables rapid appraisal of the system before conducting analysis. It is of utmost importance to tune the performance of SyftTracer before conducting the first analysis of the day.
This is facilitated through the integrated Performance Tune routine, which takes less than 10 minutes to complete, ensuring the instrument is quickly ready for accurate quantitation.
To ensure Performance Tune is executed successfully, the Syft standard gas flow must be equal to the Syft Tracer sample flow. This alignment ensures that the automatic tuning correlates with conditions that are representative of sample analysis. After completing the Performance Tune process, the SIFT-MS instrument is ready for sample analysis.
The audit trail captures essential details of the Performance Tune, including when it was executed, by whom, and its completion status. Any critical information, such as parameter values used during the flow matching process, can be tracked and entered into the audit trail using the Notes free text field.
Data files created when running the Performance Tune routine are protected and stored in the compliant database by auto-saving them in a non-editable format that cannot be deleted.
The unique file IDs of the most up-to-date steps of the Performance Tune routine are linked to successive sample analyses and included in the final PDF report of results. Copies of the results file generated via Performance Tune can be downloaded for closer analysis.
This sleek approach preserves data quality while ensuring instrument readiness and audit trail traceability, which is the cornerstone of accurate, traceable results with SyftTracer Pharm11.
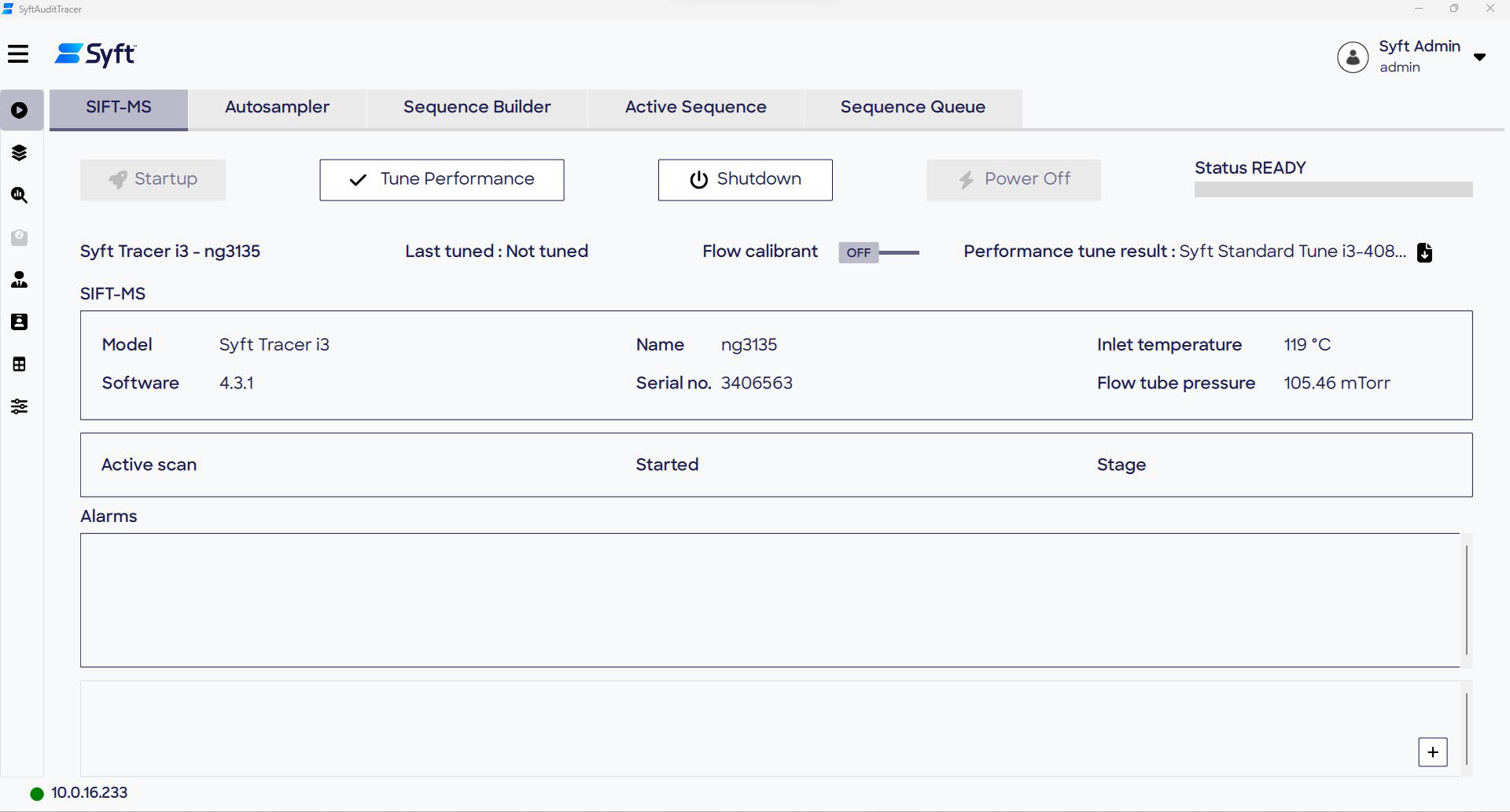
Figure 3. Instrument status information can be viewed on the SIFT-MS and Autosampler tabs of the Run page. Image Credit: Syft Technologies
Building a sequence
In the Sequence Builder tab, analytical sequences can be created and saved. Sequences provide analytical instructions for handling each sample to the autosampler and Syft Tracer instrumentation, allowing for automated sample incubation, injection, and analysis for entire batches of samples.
Information tables can be imported using the software’s CSV format, which also accommodates LIMS-exported CSV files (with requisite formatting). Sequences that have been run previously can be edited and saved under unique file IDs to create new sequences.
Each sample is assigned to a dedicated row in the Sequence Builder, which includes sample details, such as ID, location on the autosampler racks, and associated methods (SIFT-MS, autosampler).
The “Type” column determines the sample class applied in downstream data processing:
- Blank: Suitable for blank subtraction calculations, typically used for blank samples.
- Calibration: Serves as reference points for calibration; used for samples containing known analyte concentrations.
- QC: For convenience, quality control samples can be labeled, though it should be noted that no QC-specific downstream data processing is applied in SyftAuditTracer.
- Unknown: Depicts samples where target analyte concentrations are yet to be measured.
The Reference file column enables the user to select Reference files to apply to calibrant and, optionally, QC samples. Reference files specify the reference analyte concentrations in these sample types.
System administrators can configure user permissions for creating and running sequences on the Profiles page. The audit trail logs the ID of the sequence file creator and the operator who conducted the analysis and records data related to Sequence File creation.
Sequence Files and Reference Files are stored securely in the compliant database, where they can be managed in line with the standards applied to other method files in SyftAuditTracer.
Starting a sequence
Initiating the analysis is as simple as pressing the Queue Sequence button. This queues the sequence to the end of the analysis. The progress and status of a sequence can be monitored using the Active Sequence tab, providing progressive updates when running sample analysis.
Actions performed during the sequence, for instance, by the autosampler, are logged automatically in the audit trail. Once a sequence has been saved, it cannot be amended.
The analyst is able to monitor the sample analysis process in real time using LabSyft’s Live Viewer. While Live Viewer sits outside the compliant environment of SyftAuditTracer, it only offers real-time data viewing, with no editing capabilities for data or instrument settings.
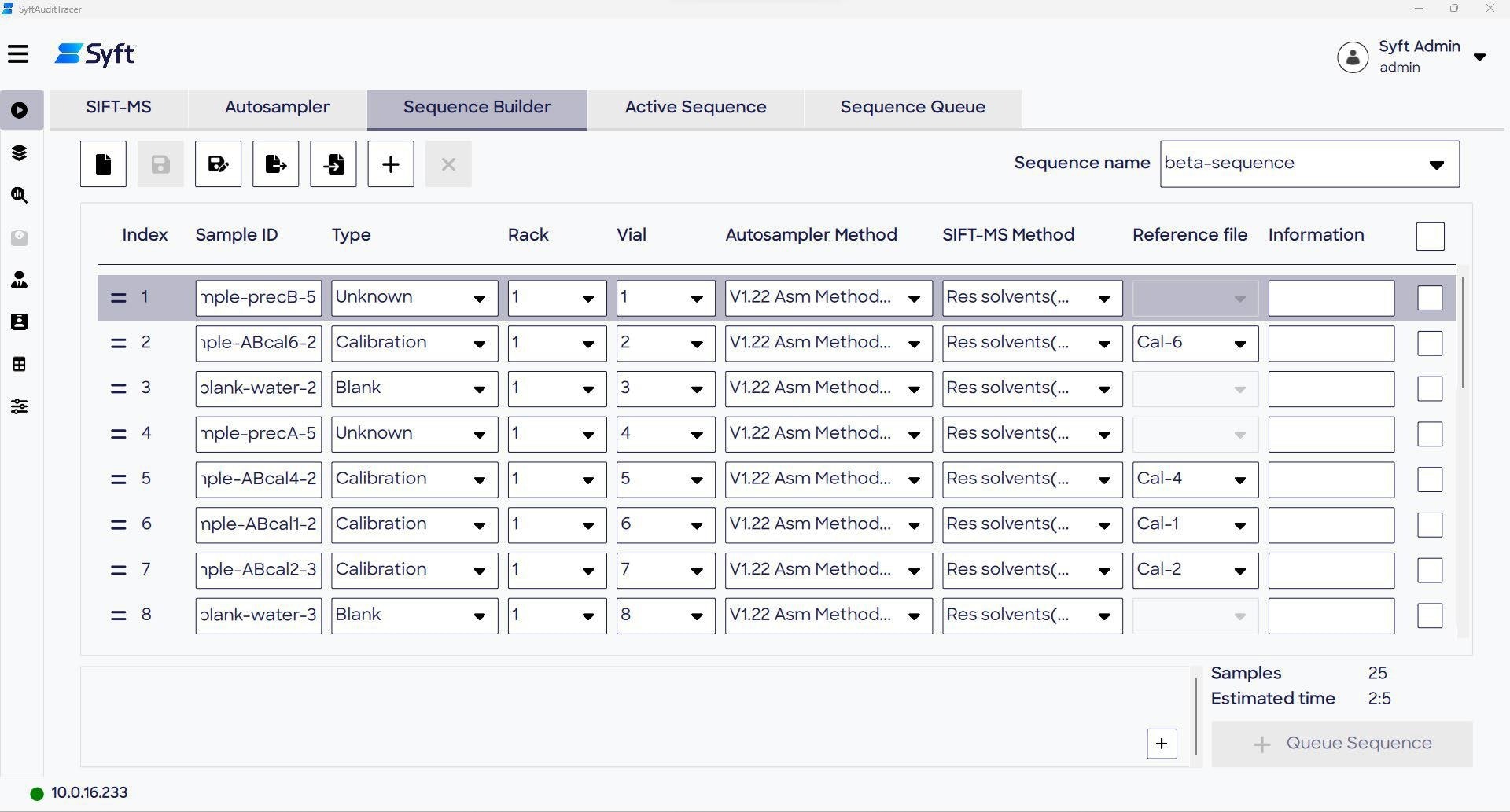
Figure 4. The Sequence Builder page of the Run screen is used to create analytical sequences that are used to conduct sample analysis. Image Credit: Syft Technologies
Amending the Sequence Queue
Additional sequences can be appended to a batch while it is running using the SyftAuditTracer.
If analysis of additional samples is required while a sequence is running, analysts have the capacity to create one or more additional sequences in the Sequence Builder which can then be queued. This flexibility is extremely useful when different analytical methods are being applied to small batch sizes.
While analysts do not have the ability to modify running sequences, they can stop or abort the Sequence Queue.
Pressing the Stop control preserves the integrity of the samples in the agitator, analyzing them before ending the sequence queue that is running. Aborting stops the entire process, which may invalidate the samples due to non-standard treatment.
Both Stop and Abort actions are recorded in the audit trail, where they can be correlated with any relevant information, such as impacted sample IDs.
Sequences queued are displayed on the Sequence Queue page of the Run screen, which includes relevant status information, Sequence ID, operator information, and timestamps. This information, in combination with user actions, system errors, and other key data, is logged in the audit trail for accountability and traceability.
Data processing
Once a sequence process is complete, the resulting data can be processed for later inclusion in an electronically signed PDF report. Processing is carried out within SyftAuditTracer and does not modify raw data files.
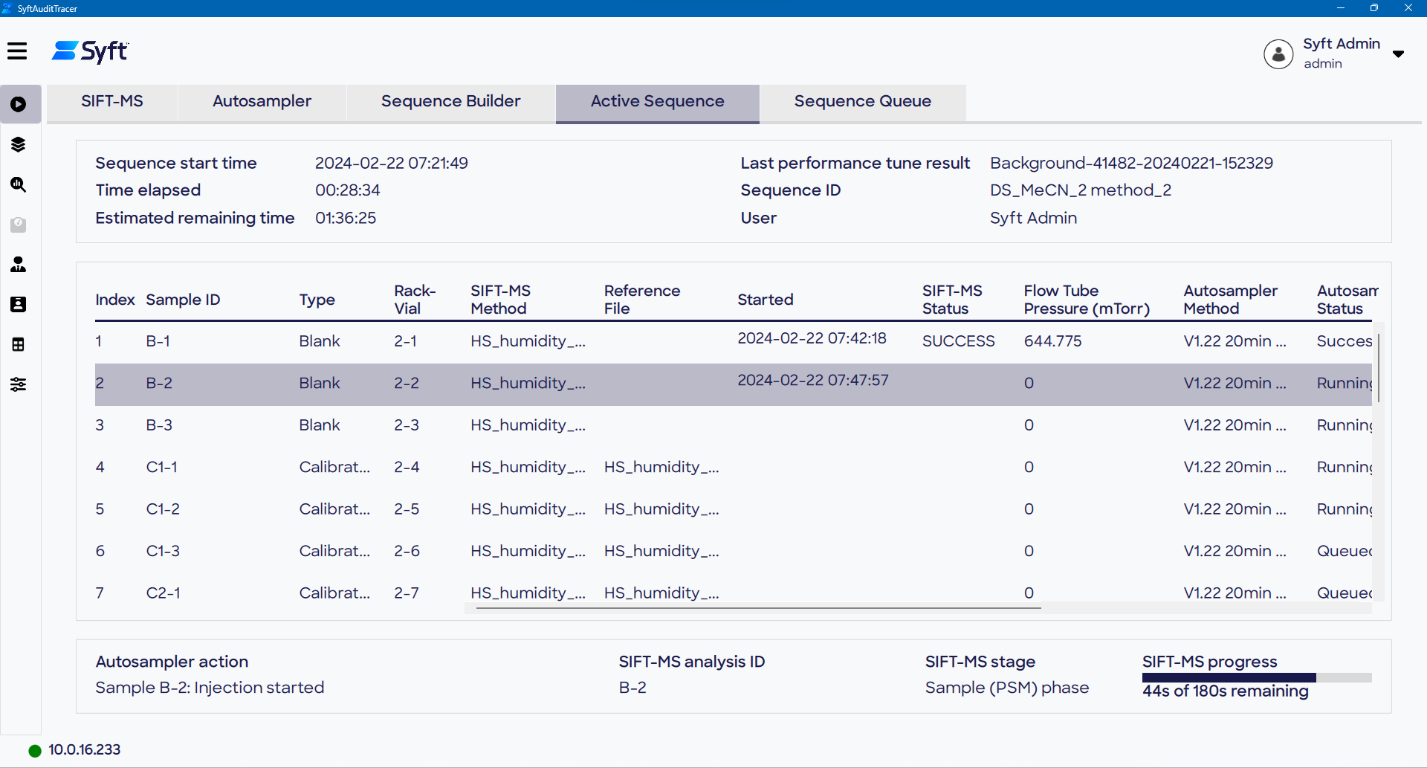
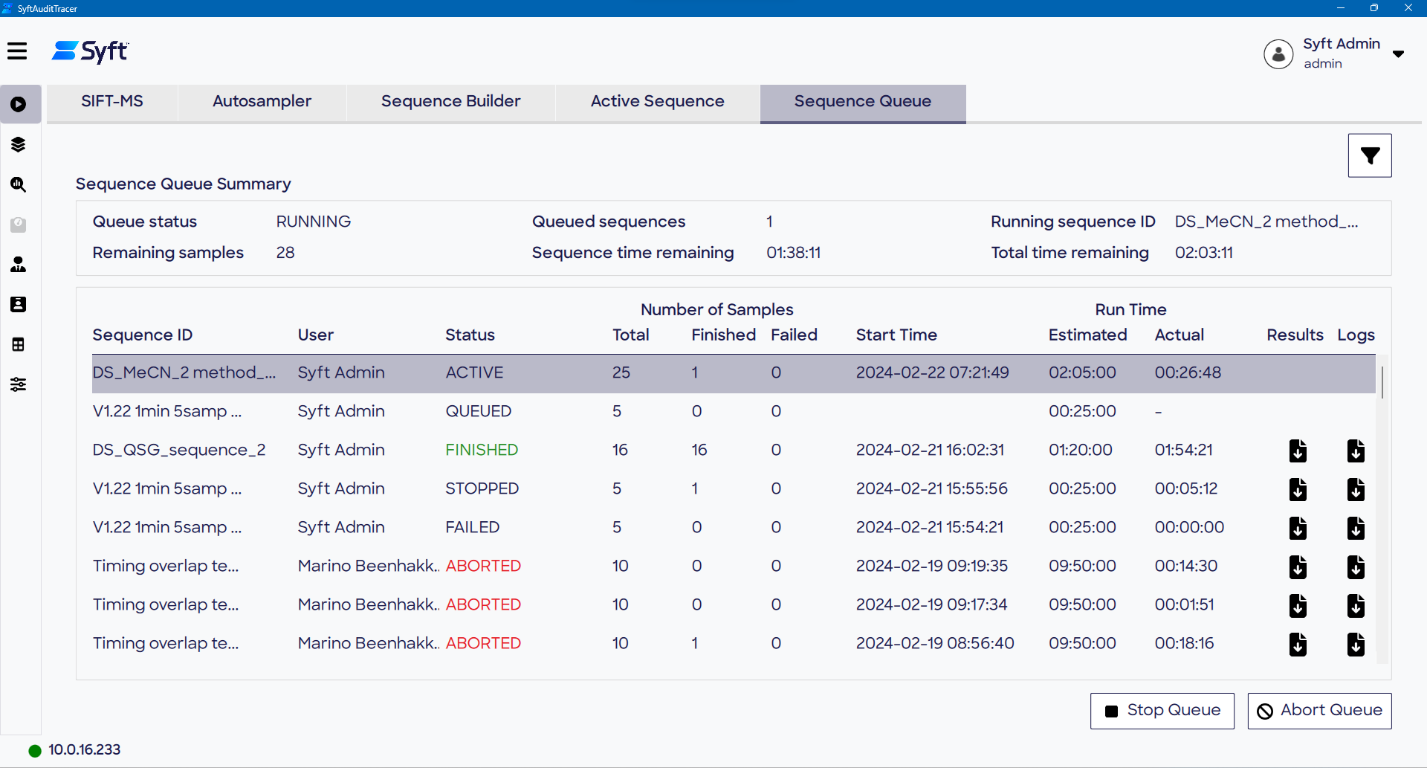
Figure 5. Active Sequence (top) and Sequence Queue (bottom) allow the progress of the current sequence and queued sequences, respectively, to be viewed. Image Credit: Syft Technologies
Data processing proceeds as follows:
- Create a data processing file
- Data quality review
- Signal processing
- Blank subtraction (optional)
- Calibration (optional)
- Reporting
In this example workflow, the assumption is that blank subtraction and calibration have been applied. SyftAuditTracer authorizes parallel data processing when running a sequence or sequence queue (note: only one user can be logged into SyftAuditTracer at a time).
Throughout parallel processing, the Sequence ID connects all data files and sample analysis data to the user who started running the sequence, making the audit trail clear.
Actions, settings, and values conducted throughout data processing and reporting processes are correlated to any important information, such as Processing File ID, sample ID, and user ID.
This data is stored in the audit trail whenever the Processing File is saved for full traceability.
Notes boxes are also available on each data processing page, which can be used to record important information or comments in text form. Entries are recorded with the date/time in the audit trail and the final report and associated with the relevant user ID.
Processing file creation
The first step of data processing involves generating a Processing File, which appears first as a draft until initiating the report signing process in the Report Approval stage. The Processing File appears on the Process Signal pages.
Processing Files can be saved and reopened in different sessions, which allows for greater flexibility as any work-in-progress can be paused and restarted later on.
Processing Files will display one of three possible statuses: Draft, Submitted, and Approved. Any editing that needs to be done during data processing can be carried out when the draft status is displayed.
Once submitted, the analyst’s electronic signature is logged against the results report, and the processing file becomes non-editable except for appending Notes for logging important comments during the review process.
Upon review and approval by a reviewer, a second electronic signature is added and stored in the compliant database as a read-only record. The Processing File includes one or multiple sequences for processing and reporting.
To make sure all sample data is accounted for, each sequence is recorded in its entirety. Traceability is ensured as each Processing File is assigned a unique ID and associated with the user who created it. Only the original creator has the permissions to make changes to the Processing File.
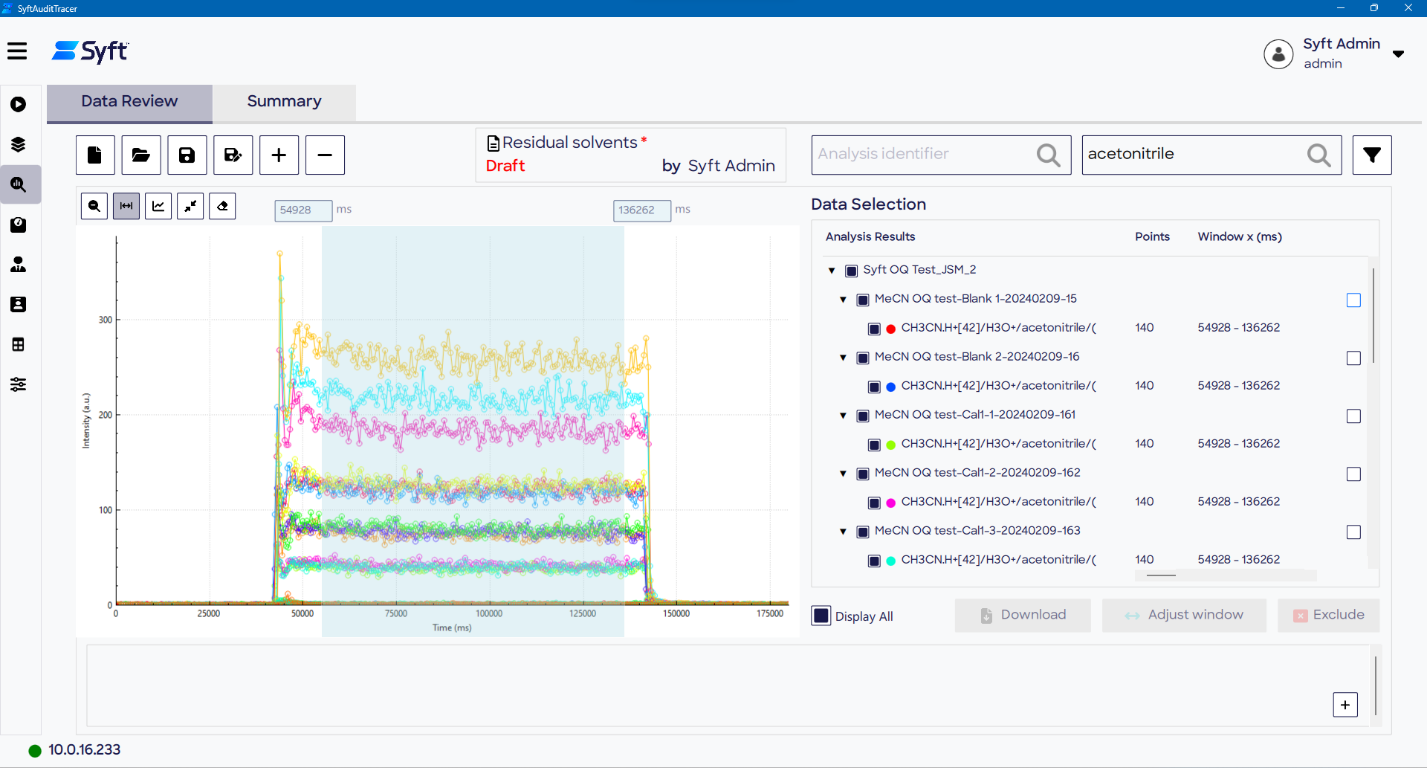
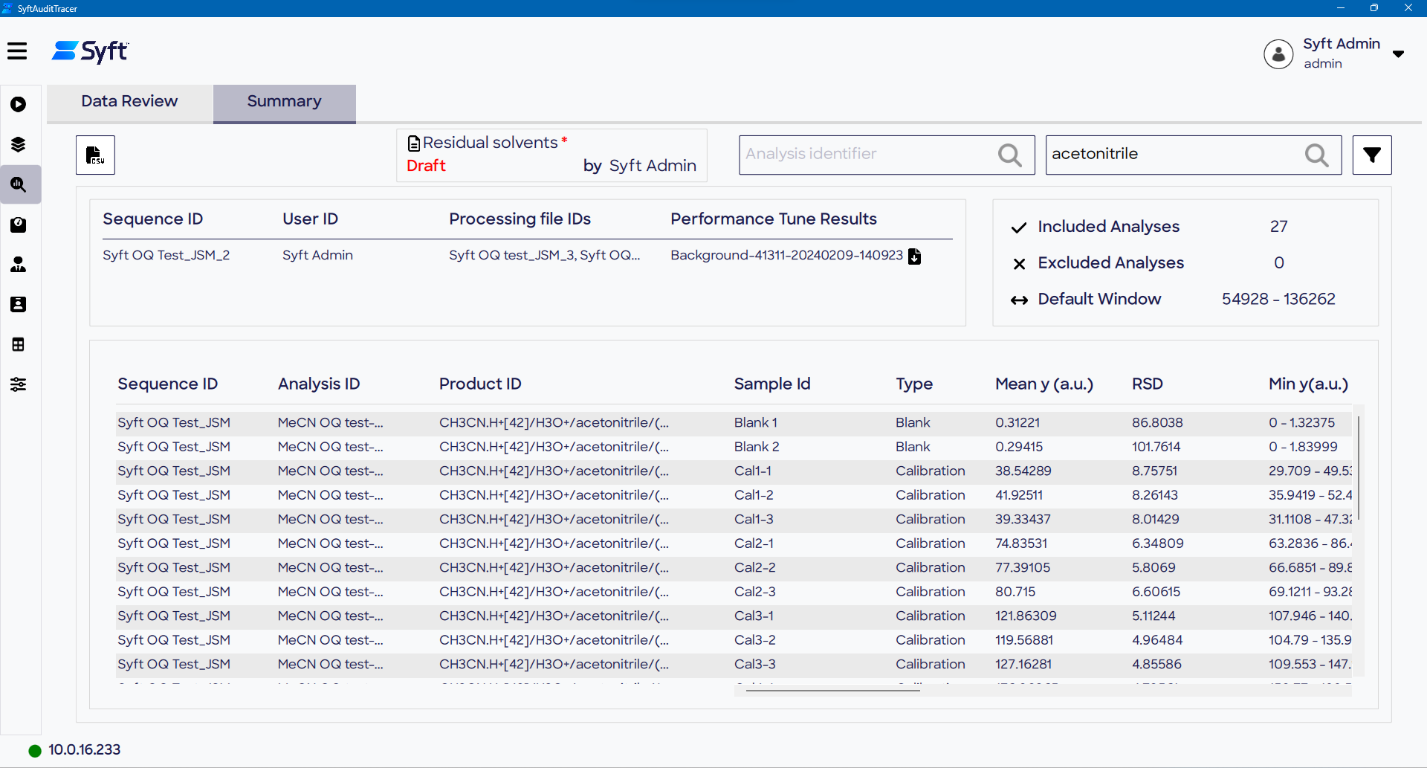
Figure 6. Data review and signal processing are performed on the Data Review (top) and Summary (bottom) pages of the Process Signal screen. Image Credit: Syft Technologies
Data quality review
Before conducting any data processing calculations, a review of the data quality should be conducted on the Data Review page of the Process Signal screen. This process is streamlined using customizable filters, pan and zoom.
In the event of sample failure, the option to omit problematic data is available, with relevant filenames and the reason for exclusion included in the audit trail and PDF report.
Signal processing
After a thorough review, the samples are ready for signal processing and automated headspace analysis with SyftAuditTracer.
The raw signal is processed in automated headspace analysis with SyftAuditTracer by calculating the mean signal intensity over a user-defined time “window.” Upon the rare occasion where a shift in the injection peak position occurs, the window for the shifted peak(s) can be ameneded as long as the SOP permits this.
Samples that have had their windows modified are documented in the final PDF report and the audit trail, specifying the adjusted window (start and end time in seconds) and the reason for the modification.
Summary statistics across the window (mean, standard deviation, %RSD, n points) can be viewed per product once the averaging windows have been determined.
In Syft Tracer analyses, the recommended default approach for quantitation is to select one product per analyte for use as a “quantifier,” with additional products for the same analyte used as “qualifiers.” The mean intensity values are then carried over to downstream data processing.
A CSV file that contains signal-processed data can be exported and uploaded to alternative software systems for custom data processing. The export of signal-processed data is recorded in the audit trail alongside the unique file ID of the CSV file for traceability.
Blank subtraction
Data from blank samples are viewable on the Blank Subtract page of the Quantitate screen. Blanks to be added to the subtraction calculation which have been determined in line with an SOP, are selected. Blank mean and standard deviation are calculated across the blank samples chosen for each product ion.
When toggling the “Apply Blank Subtraction”, these values are automatically subtracted from all non-blank data in the batch.
Calibration
During the calibration process, a calibration is assigned to quantify known samples. Calibrations are constructed on the Calibration page, with a variety of options available:
- From Curve: Calibration curves are constructed across multiple concentrations per product, utilizing calibrant samples from the data processing file. Conversely, it can be used for single-point calibration (to give a quantitation factor) per product.
- Custom: Facilitates manual data entry of slope and intercept per product.
- From previous: A calibration file from a previous analysis can be applied, where either 1 or 2 above have been selected for each product ion, respectively. Calibration files are controlled per the 21 CFR Part 11 standards applied to other files in SyftAuditTracer. For instance, it is not possible to edit or delete files.
Which method to apply (between options 1 and 2) can be configured per product ion. Alternatively, when option 3 is selected, the calibrations present in the previous Calibration file are applied across the data for all product ions in the analytical batch.
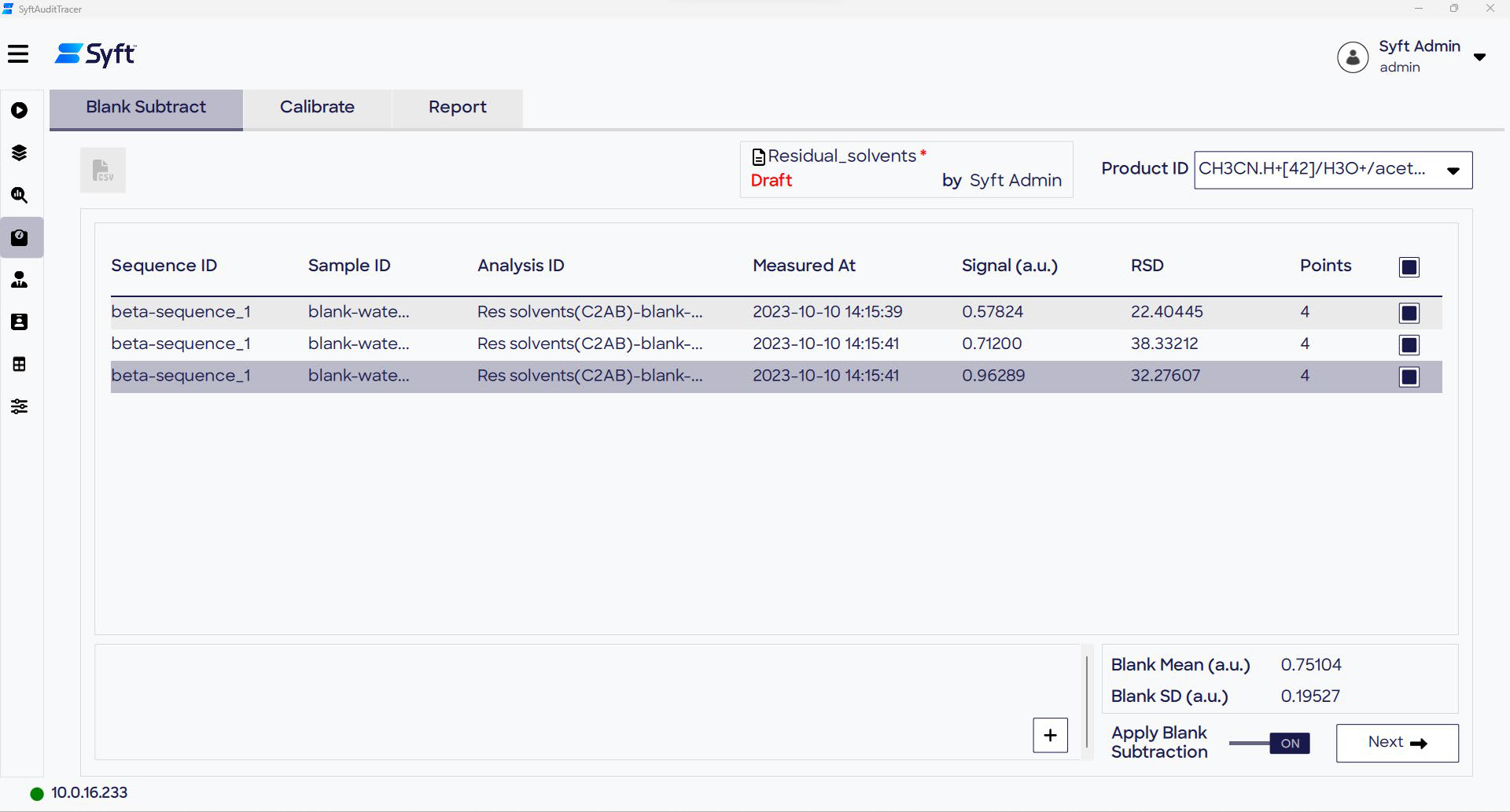
Figure 7. The Blank Subtract page of the Quantitate screen provides the option to subtract blank sample data from other types of sample data. Image Credit: Syft Technologies
In this example, it has been assumed that a calibration curve has been included in the batch.
A calibration curve is created per product utilizing the sample data of the calibrant. A Quantitation SOP should determine which products are being subjected to quantitation alongside quantitation methodology. The calibration data can be selected for inclusion in the curve from calibrant samples in the batch, which are displayed automatically in the table.
To create a calibration curve, “From Curve” is selected. If necessary, the calibration relationship can be forced through zero. The relevant data points and regression line are displayed in a graph plotting the reference concentration against the measured signal.
The audit trail and report also account for the calculated slope and intercept for the regression, with additional statistical measures. The calibration is used to quantify the remaining data, which comprises Unknown and QC sample types.
Reporting
Final reports are created on the Report page of the Quantitate screen. Moreover, all data processing steps, intermediate values, and the final concentration (Sample Conc.) are included in a summary report.
The report table readily displays key information such as Sample ID, Sequence ID, raw data file ID (analysis ID), and Method ID. A CSV report table can be exported for external editing or uploaded and stored in a Laboratory Information Management System (LIMS), although reformatting may be required.
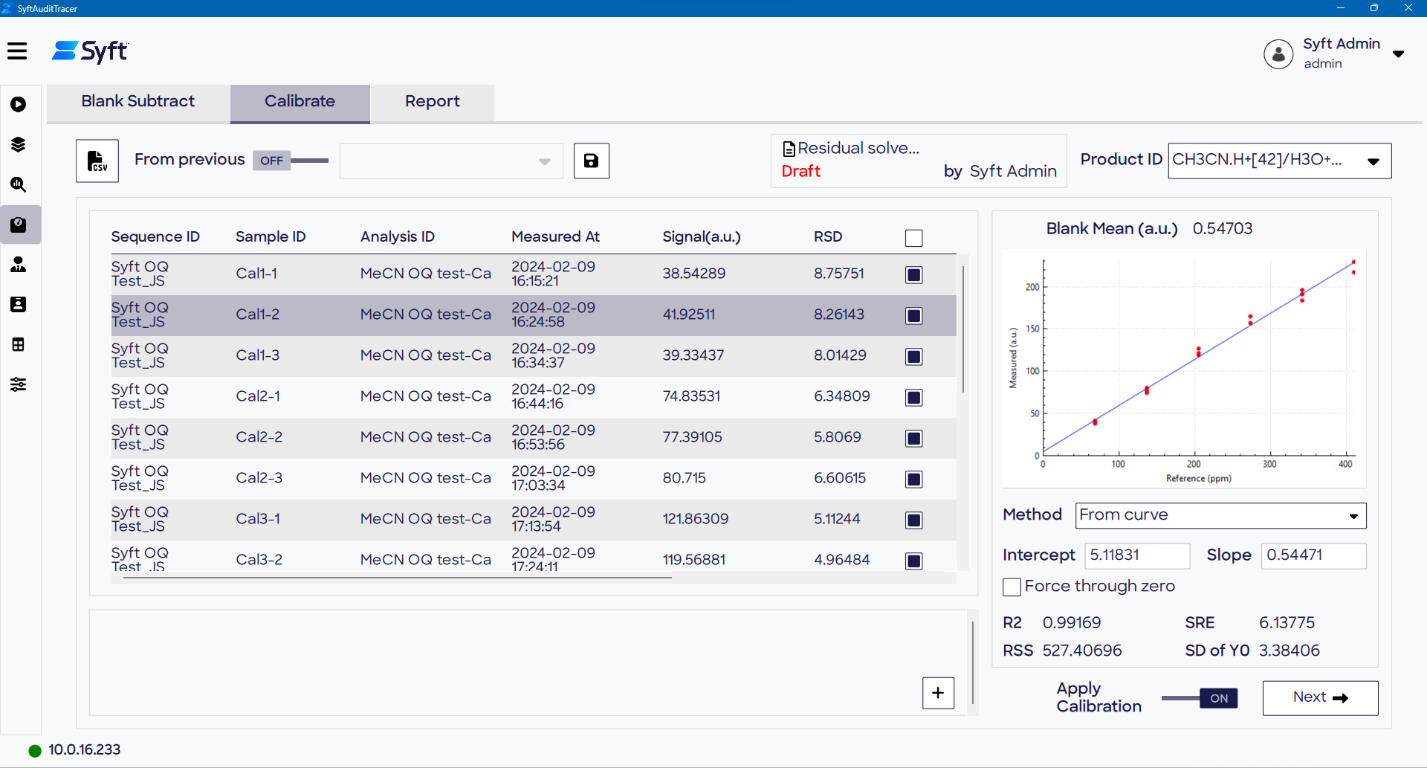
Figure 8. The option to calibrate using various methods is provided on the Calibration page. Image Credit: Syft Technologies
After each of the applied steps has been reviewed, the draft report can be submitted for approval, thereby initiating the reporting process.
Whoever created the report uses their username and password to sign the report electronically. A second reviewer then logs in and can approve the submission and add their electronic signature using their login credentials.
This process produces an electronically signed PDF report to comply with 21 CFR Part 11, which is recorded in the compliant database in a non-editable format. If the second reviewer rejects the report, the first analyst can make the required adjustments before resubmitting for approval.
Each iteration of the approval process requires successive logouts and logins, with the first user logging out after submission and the reviewer logging in for approval. The audit trail tracks and records all login/logout activity, including User ID, time and date, and actions during the approval process.
The PDF reports, which include all relevant information, are generated in draft, submitted, or approved statuses and ready for download by users with the requisite permissions.
Draft versions can be generated prior to initiating the approval process. Submitted reports are generated upon each submission, and the final, approved PDF report is created once approval is complete.
It is only possible to edit the draft Processing Files with other versions locked. PDF copies of submitted and approved report versions are automatically generated, while users with the correct permissions can create draft reports.
All locked PDF reports that have been created are protected and stored as records, with unique IDs that combine the Report title (input by the analyst), report status, and date/time stamp to prevent editing.
After reporting
Once approval of the PDF report is granted, the processing and reporting tasks for this dataset are marked as complete. While viewable, the Processing File and final signed PDF report are locked and cannot be edited.
The source data, stored in the raw data files, can be accessed for future inclusion in new Processing Files (each with a unique ID) and reports, as and when the laboratory requires.
Crucially, re-reporting or re-processing data a number of times can be traced and distinguished as unique IDs are assigned to each Processing File. This ensures complete documentation and auditability of the whole data lifecycle.
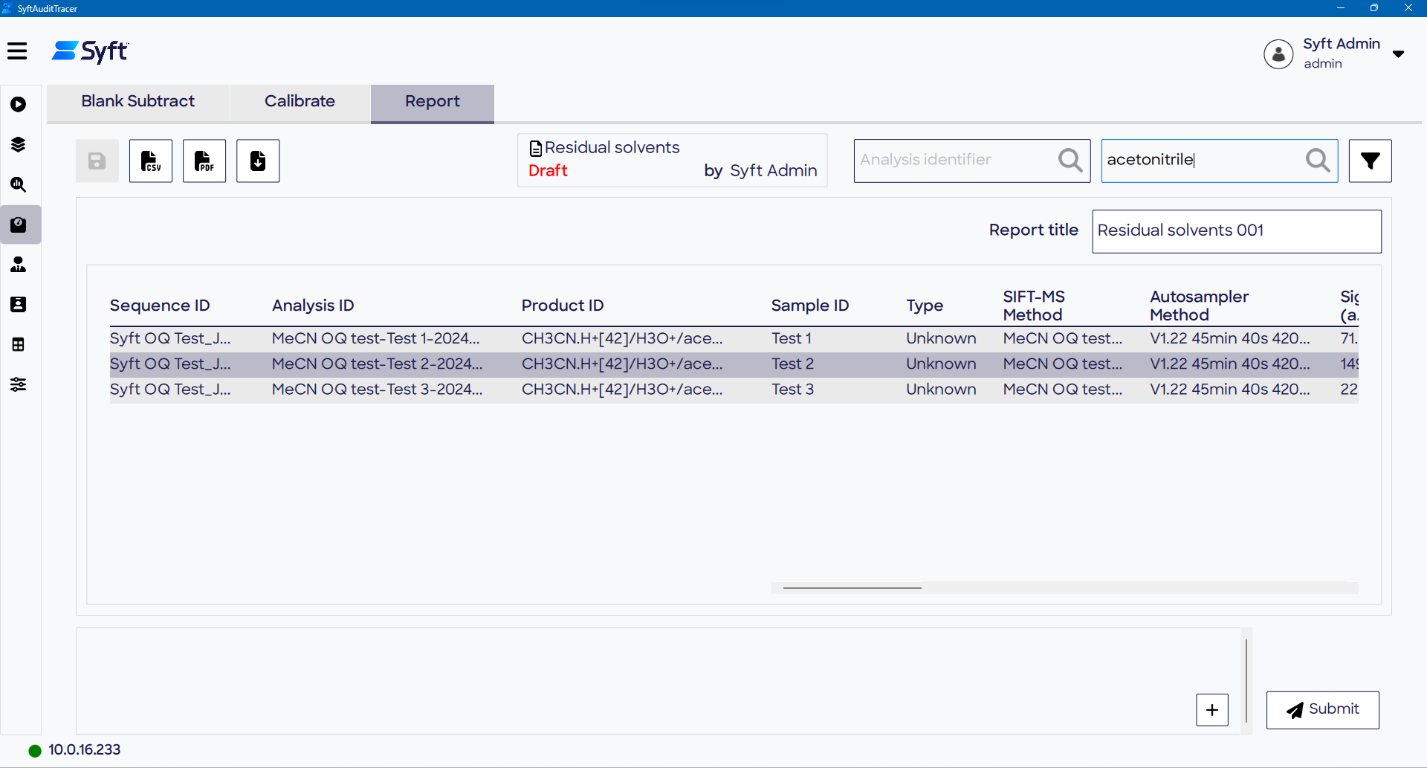
Figure 9. Key information is displayed in the table on the Report page of the Quantitate screen, from where reports can be generated. Image Credit: Syft Technologies
Conclusion
This overview of the Syft Tracer Pharm11 analytical workflow, SyftAuditTracer software, and associated record management highlights its comprehensive support for 21 CFR Part 11 requirements.
The SyftAuditTracer software, with its advanced, novel user roles and permissions, grants controlled access to advanced capabilities with comprehensive administrative features, bringing the rapid, user-friendly SIFT-MS to the regulated pharmaceutical laboratory for the first time.
The analytical workflow enables customization while complying with the most robust data integrity standards.
From user login to the final PDF report, traceability, security, and auditability are detailed throughout. Files crucial to analytical accuracy are managed to ensure their integrity. Meanwhile, the audit trail tracks user actions, which allows for a clear chain of custody for operations conducted in the software.
The electronic signature approval process clearly established a good working compliance framework, resulting in a final, encrypted, read-only PDF report of analytical results.
SyftAuditTracer allows laboratories to comply with the stringent regulatory demands of 21 CFR Part 11 via a user-friendly and flexible interface. Syft Tracer Pharm11 provides the pharmaceutical industry with a powerful solution for overcoming its most critical analytical challenges.
References
- U. S. Food and Drug Administration (2003). Guidance for Industry Part 11, Electronic Records; Electronic Signatures—Scope and Application. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/part-11-electronic-records-electronic-signatures-scope-and-application
- U. S. Food and Drug Administration (1997). 21 CFR Part 11. Electronic Records; Electronic Signatures; Final Rule Electronic Submissions; Establishment of Public Docket; Notice.
- Langford VS, Perkins MJ (2023). Syft Tracer™: Next-generation volatile impurity analysis for enhanced workflows. Syft Technologies application note. http://bit.ly/3LYH2IP
- Langford VS (2023). SIFT-MS: Quantifying the volatiles you smell… and the toxics you don’t. Chemosensors 11, 111. https://doi.org/10.3390/chemosensors11020111
- Smith D, Španěl P, Demarais N, Langford VS, McEwan MJ (2023). Recent developments and applications of selected ion flow tube mass spectrometry (SIFT-MS). Mass Spec. Rev. e21835. https://doi.org/10.1002/mas.21835
About Syft Technologies
Syft Technologies is the world leader in real-time, direct injection mass spectrometry with more than 20 years of SIFT-MS expertise. The unique attributes of Selected Ion Flow Tube Mass Spectrometry (SIFT-MS) have enabled our customers to remove bottlenecks and solve daunting analytical challenges. The results are improved capacity and organizational efficiency for our customers and a safer world.
Syft Technologies supports a broad range of worldwide industries including semiconductor manufacturing, pharma and CDMOs, environmental protection, automotive, food, flavor and fragrance, and many more. Syft has offices throughout the world offering 24/7 service and support including those in New Zealand, Korea, Taiwan, Singapore, Germany and the U.S.
SIFT-MS delivers real-time, chromatography-free direct analysis of compounds that traditionally require intensive sample preparation. No sample preparation is required for even complex matrices and high-humidity samples. SIFT-MS analyzes compounds that cannot be easily targeted by traditional chromatographic methods such as formaldehyde, hydrogen sulfide, ammonia, ethylene oxide, and nitrosamines. SIFT-MS is industry-proven, providing non-technical operators with the laboratory-grade chemical analysis presented in a format that they can easily understand and act on. Syft’s flagship product is the Syft Tracer which delivers routine and repeatable analysis of volatile compounds.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.