- Reduces the duration of ELISA experimentation from 260 minutes to 90 minutes without compromising sensitivity or reproducibility using Abcam’s SimpleStep ELISA kit.
- Reads a full ELISA plate in just five seconds using the SpectraMax ABS Plus Microplate Reader.
- Automatically calculates sample concentrations and plots results using SoftMax Pro Software.
Introduction
THP-1, a human cell line originating from a leukemia patient, is widely used as a model to study macrophage function in vitro. Typically cultured in suspension, THP-1 cells differentiate into macrophages and become adherent when treated with phorbol 12-myristate 13-acetate (PMA). They also start releasing cytokines, including interleukin-8 (IL-8), which is secreted by various cell types and contributes to the regulation of the acute inflammatory response.
Enzyme-linked immunosorbent assays (ELISAs) are routinely used to quantify analytes such as IL-8 within a 96-well microplate format. A standard ELISA generally requires more than four hours to perform. Abcam’s SimpleStep ELISA® cuts that time to just 90 minutes, while maintaining sensitivity and reproducibility (Figure 1).
The following procedure describes how to operate the Human IL-8 SimpleStep ELISA and detect the results using the SpectraMax® ABS Plus Microplate Reader, using an effective preconfigured protocol in SoftMax® Pro Software.
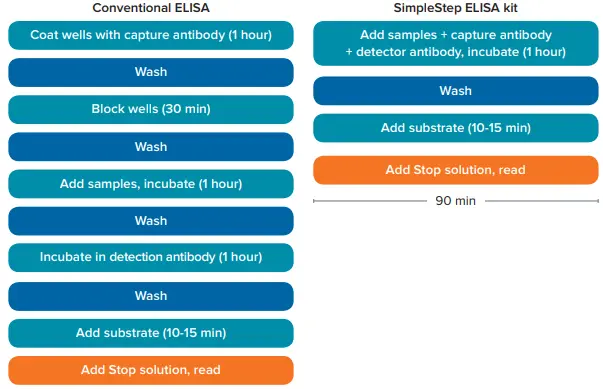
Figure 1. Comparison between SimpleStep ELISA kit and conventional ELISA workflows. Abcam’s SimpleStep ELISA kit provides results in 90 minutes with its streamlined protocol. Unlike conventional ELISAs, the SimpleStep ELISA kit only requires one incubation and one wash step, reducing assay time by nearly two-thirds. Image Credit: Molecular Devices UK Ltd
Materials
- Human IL-8 SimpleStep ELISA kit (Abcam cat. #ab214030)
- THP-1 human peripheral blood monocytes (ATCC cat. #TIB-202)
- THP-1 culture medium
- RPMI-1640 Medium (ATCC cat. #30-2001)
- Benchmark Fetal Bovine Serum (Gemini cat. #100-106)
- 2-Mercaptoethanol (Sigma cat. #M6250-100mL)
- Penicillin-Streptomycin (10,000 U/mL) (ThermoFisher cat. #15140122)
- SpectraMax ABS Plus Microplate Reader (Molecular Devices cat. #ABS Plus)
Methods
THP-1 cell preparation
20,000 undifferentiated THP-1 suspension cells were seeded into each well of a 96-well microplate. The THP-1 cells were then treated with 2.5 ng/µL PMA or DMSO (negative control), diluted in culture media. After treatment, the cells were incubated at 37 °C for 48 hours. Cell supernatants were collected and stored at -80 °C until the ELISA was performed.
ELISA
On the day of the ELISA, sample supernatants were thawed, spun down at 2,000 x g for 10 minutes using a micro-centrifuge to eliminate debris, and diluted at ratios of 1:5, 1:10, and 1:50 using the supplied Sample Diluent.
The Human IL-8 SimpleStep ELISA reagents and standards were prepared according to the kit’s Protocol Booklet. Samples and standards were added to the supplied microplate, followed by the addition of the Antibody Cocktail to the wells. The plate was then sealed and incubated for one hour at ambient temperature on a plate shaker.
The wells were washed three times with Wash Buffer PT buffer to remove unbound material. TMB Substrate was added to each well, and the plate was incubated for 15 minutes in the dark on a plate shaker.
Stop Solution was added to each well, and absorbance (OD) was measured at 450 nm on the SpectraMax ABS Plus Microplate Reader with a preconfigured protocol in SoftMax Pro Software (refer to Figure 1 for workflow).
Results
The assay produced a linear IL-8 standard curve with r2 = 1.000, using the 'HRP and TMB' protocol in SoftMax Pro Software (Figure 2). The software used this curve to interpolate IL-8 concentrations of samples derived from DMSO-treated negative control cells and PMA-treated cells.
Samples lacking sufficient dilution were identified in the group table of the preconfigured protocol as out of range ('R') of the standard curve (Figure 3). PMA-treated cells released 3,400 pg/mL of IL-8, around 55 times more than the 63 pg/mL seen in untreated controls. (Figure 4).
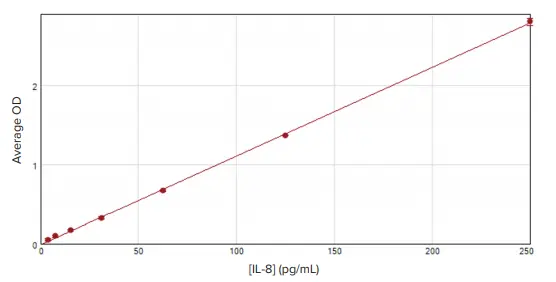
Figure 2. ELISA standard curve. The IL-8 standards were diluted in Sample Diluent and assayed using the Human IL-8 SimpleStep ELISA kit. A linear curve fit was applied to the standards in SoftMax Pro Software, and the generated standard curve demonstrated excellent linearity (r2 = 1.000). Image Credit: Molecular Devices UK Ltd
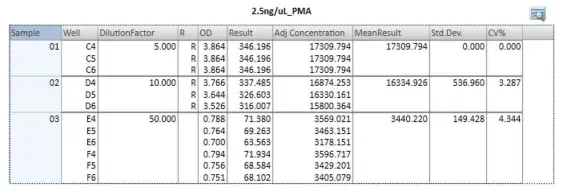
Figure 3. Group table displaying calculated sample concentrations. The IL-8 concentrations of PMA-treated cells were automatically interpolated from the standard curve by the preconfigured SoftMax Pro protocol and displayed in a group table. Any samples showing an ‘R’ in the ‘R’ column had a calculated concentration that is outside the range of the standard curve, in this case indicating the need to dilute the sample further. Image Credit: Molecular Devices UK Ltd
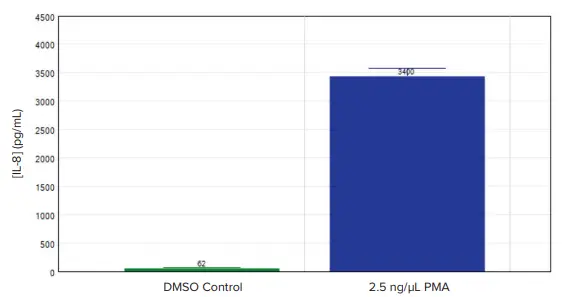
Figure 4. IL-8 concentrations measured in undifferentiated (DMSO control) and differentiated (2.5 ng/µL PMA) THP-1 cells. 2.5 ng/µL PMA-treated THP-1 cells produced 3400 pg/mL of IL-8 compared to the 62 pg/mL IL-8 produced by undifferentiated THP-1 cells, a 55-fold increase. Image Credit: Molecular Devices UK Ltd
Conclusion
The SpectraMax ABS Plus Microplate Reader enables fast and sensitive IL-8 detection with Abcam’s Human IL-8 SimpleStep ELISA kit. Using proprietary antibody binding technology, the SimpleStep ELISA enables IL-8 quantification in THP-1 cell supernatant in only 90 minutes, approximately one-third of the time necessary to conduct a traditional ELISA, without compromising sensitivity or reproducibility.
The reader’s eight-channel detector can read a full 96-well ELISA plate in only five seconds. A preconfigured protocol in SoftMax Pro Software automatically plots a standard curve, determines sample concentrations, and plots results for negative controls versus treated cells, further minimizing the time required to achieve publication-quality results.
Acknowledgements
Produced from materials originally authored by Cathy Olsen, Sr. Applications Scientist at Molecular Devices.
About Molecular Devices UK Ltd
Molecular Devices is one of the world’s leading providers of high-performance bioanalytical measurement systems, software, and consumables for life science research, pharmaceutical, and biotherapeutic development. Included within a broad product portfolio are platforms for high-throughput screening, genomic and cellular analysis, colony selection, and microplate detection. These leading-edge products enable scientists to improve productivity and effectiveness, ultimately accelerating research and the discovery of new therapeutics. Molecular Devices is committed to the continual development of innovative solutions for life science applications. The company is headquartered in Silicon Valley, California, with offices around the globe. For more information, please visit www.moleculardevices.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.