Sponsored Content by AbselionReviewed by Olivia FrostNov 28 2025
Monoclonal antibodies (mAbs) are still one of the most commonly employed therapeutic modalities in oncology, autoimmune illnesses, and infectious diseases. In upstream development, precise and fast measurement of mAb concentration is critical for clone selection, process optimization, and early screening workflows.
Conventional procedures such as enzyme-linked immunosorbent tests (ELISA) and high-performance liquid chromatography (HPLC) provide trustworthy results, but they can necessitate lengthy processes, specialized instrumentation, and matrix cleaning.
The Amperia™ platform allows for consistent, sensor-based mAb quantification straight from cell culture supernatants using an inverse occupancy assay methodology. It has flexible throughput and a reduced setup process.
Assay format and workflow
Amperia™ quantifies mAbs using an inverse occupancy format. Each dip-style sensor is precoated with either Protein A or Protein G, which captures antibodies' Fc regions during the sample incubation process.
Protein A has a high affinity for human IgG subclasses, but Protein G covers a wider range of species, including mouse IgG1. The choice of sensor is determined by the antibody source and assay needs.
Following incubation, a labeled detection molecule is introduced, which also binds to accessible Fc-binding sites on the sensor surface. As antibody concentration grows, the analyte occupies more binding sites, lowering the availability of detecting molecules and resulting in a reduced signal.
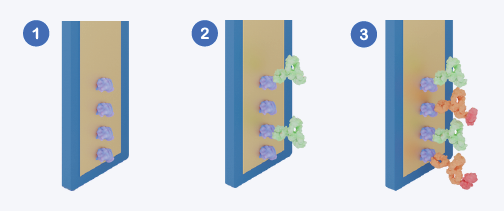
Assay Workflow Schematic. (1) Sensor surface coated with Protein A or Protein G. (2) Antibody (analyte) binds via Fc interaction. (3) Detection molecule binds remaining unoccupied Fc-binding sites, generating an inverse signal. Image Credit: Abselion
This inverse signal behavior allows for precise measurement across a large dynamic range, including crude culture medium.
Assays are conducted through the system's touchscreen-guided workflow, which includes built-in protocol templates that cover common run combinations. Amperia's straightforward setup and integrated analysis allow for streamlined and reproducible mAb quantification.
Source: Abselion
| Parameter |
Typical Value |
| Sample throughput |
Up to 64 samples per run |
| Total run time |
~ 75 - 200 min |
| Hands-on time |
~ 15 - 30 min |
| Detection Range |
Protein A Assay: High: 1.88 - 240 μg/ml;
Mid: 0.23 - 30 μg/ml; Low: 0.05 - 6.4 μg/ml |
Protein G Assay: μg/mL‑scale;
Range varies by isotype/subclass and affinity |
| Format |
Inverse Occupancy (Protein A/Protein G capture) |
Note: Values shown are typical ranges based on internal testing. Actual throughput and hands-on time may vary depending on assay format, sample type, and workflow configuration. Detection range may vary depending on analyte affinity and assay conditions.
Data highlights
Amperia™ provides accurate antibody measurement using Protein A and Protein G, with performance in high, mid, and low ranges. The format accommodates a wide range of species and subtypes, including humans and mice, ensuring consistent findings throughout research and development.
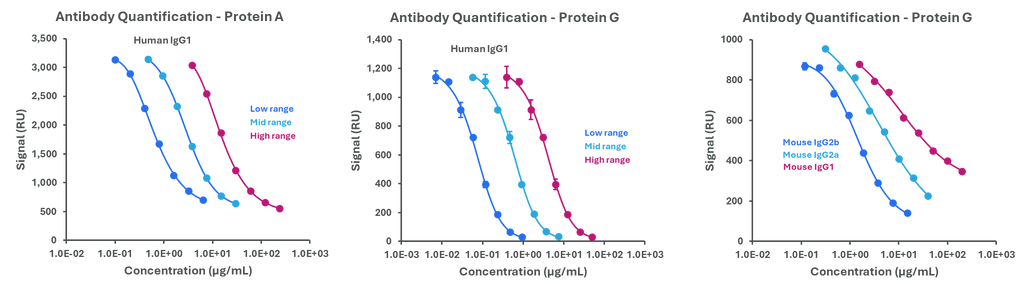
Representative standard curves for Protein A and Protein G assays. Image Credit: Abselion
Where Amperia supports mAb workflows
Amperia™ enables various mAb development approaches, especially in early upstream contexts that prioritize speed and scalability. With constant performance in primitive matrices and simple setup, it allows for rapid iteration across many use cases.
Source: Abselion
| Use Case |
Typical Sample Type |
Value |
| Clone ranking |
Cell culture supernatant |
Rapid screening across candidates |
| Process optimization |
Supernatant from fed-batch runs |
Track titers under changing conditions |
| Media comparison |
Unpurified production media |
Assess yield and stability |
| Expression system evaluation |
Various host cells |
Compare production across systems |
Summary
Amperia™ offers a scalable solution for mAb quantification through sensor-based detection, guided procedures, and broad matrix compatibility. It is ideal for early-stage process development, providing a viable alternative to standard assays when speed, simplicity, and repeatability are critical.
About Abselion
Abselion started in 2018, at that time under the name HexagonFab, in a small corner of a laboratory at the University of Cambridge.
We set out with the humble goal to make protein research simpler. Scientists should be able to pursue their passion for discovery and innovation, rather than spend their valuable time with tedious, manual tasks. With RED we had access to the ideal technology to create this product. A product that is so compact that it could fit on every bench, and so affordable that it is accessible to everyone. Over the years we have designed, built and tested our first product Amperia and we’re proud to introduce it to the world.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.