Sponsored Content by AbselionReviewed by Olivia FrostNov 28 2025
His-tagged proteins underpin research in structural biology, test development, and drug development. Their small tag enables easy purification and rapid construct iteration. When screening many constructions, teams want rapid, quantitative readouts from crude lysates to determine what to grow and what to discard.
Standard methods like SDS-PAGE and spectrophotometry are still useful, although they can lead to uncertainty at the early stages. The Amperia™ platform's His-tag assay allows teams to obtain reproducible concentration data straight from unpurified samples, guiding expression and purification decisions upfront.
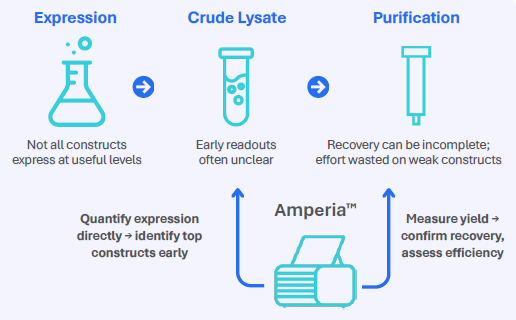
Fig. 1. Amperia provides quantitative insight in expression screening. Image Credit: Abselion
Image Credit: Abselion
About the assay
The Amperia™ His-tag assay employs a premix competition format, where His-tagged proteins in the sample compete with a labeled His-tagged detection reagent for binding sites. This inverse signal connection allows for precise measurement across a wide dynamic range, including crude lysates.
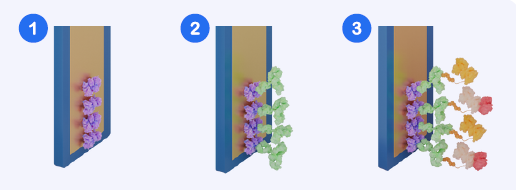
Fig. 2. Assay Workflow Schematic. (1) Sensor with streptavidin surface. (2) Biotinylated anti-His antibody bound to sensor. (3) Premixed sample and HRP-labelled His-tagged detection reagent applied, enabling signal generation. Note: In the commercial kit, steps 1 & 2 are completed during manufacturing. Image Credit: Abselion
Project context
A global pharmaceutical company evaluated eight His-tagged protein creations in E. coli using the Amperia™ platform.
Each construct contained an 8-His tag. Cells were lysed, and crude lysates were examined directly, with corresponding purified fractions available for comparison. The target protein's molecular weight was around 30 kDa.
Results
Screening crude lysates
Eight constructs (S1–S8) were produced and evaluated as crude lysates. On SDS-PAGE (Fig. 3 top left), several samples (S1-S6) had strong but overlapping bands, but others (S7, S8) had no discernible band at the 30 kDa target. Interpreting relative yields proved problematic.
Amperia produced a clear ranking in a single run (Fig. 3, top right, blue). S4 had the greatest expression level (~2230 µg/mL), followed by S5 (~670 µg/mL) and S1 (~400 µg/mL). S2 and S6 produced modest signals, whilst the remaining constructions displayed little or no expression.
Analyzing purified fractions
Samples were purified by spin columns (200 µL crude → two 200 µL elutions).
SDS-PAGE (Fig. 3 bottom) revealed distinct bands at around 30 kDa, with S4 being the strongest. Other designs yielded no discernible bands.
Amperia corroborated the same ranking in the first elution (Fig. 3 top right, red), measuring around 750 µg/mL for S4, 150 µg/mL for S5, and 120 µg/mL for S1. Other constructs were at or near the baseline.
Concentrations in the first elution were lower than those seen in crude lysates. Additional protein was seen in the gel's second elution (Fig. 3 bottom), indicating partial resin recovery.
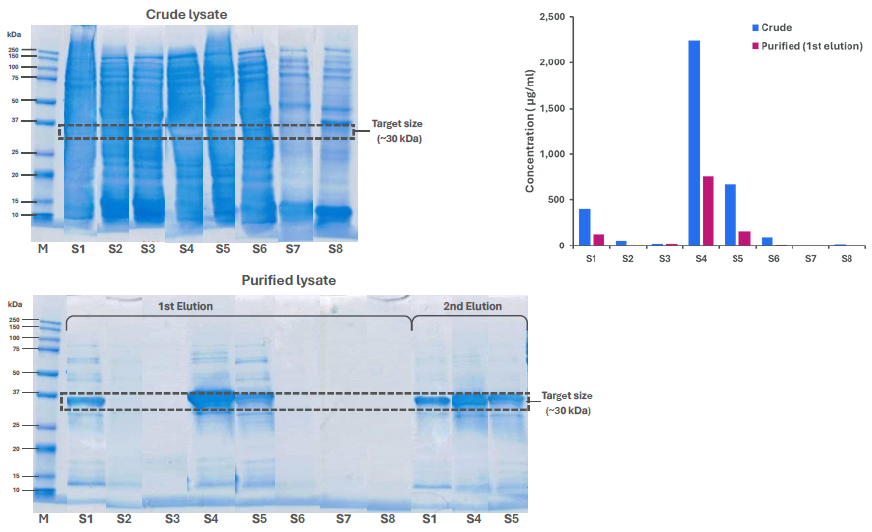
Fig. 3. Crude lysates and purified fractions analysed by SDS-PAGE and Amperia. Amperia provided quantitative ranking in crude and 1st elution samples, while SDS-PAGE also showed protein in the 2nd elution. Image Credit: Abselion
Workflow insights
Amperia provided a clear construct ranking in crude lysates (S4 > S5 > S1), whereas gels were confusing. The same order was confirmed following purification, albeit at lower quantities, and gels revealed protein in the second elution.
Together, these findings demonstrated how Amperia may provide clear, quantitative insights on His-tag protein expression, allowing for more informed downstream decisions.
About Abselion
Abselion started in 2018, at that time under the name HexagonFab, in a small corner of a laboratory at the University of Cambridge.
We set out with the humble goal to make protein research simpler. Scientists should be able to pursue their passion for discovery and innovation, rather than spend their valuable time with tedious, manual tasks. With RED we had access to the ideal technology to create this product. A product that is so compact that it could fit on every bench, and so affordable that it is accessible to everyone. Over the years we have designed, built and tested our first product Amperia and we’re proud to introduce it to the world.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.