Adeno-associated viruses (AAVs) have transformed the landscape of gene therapy product development. In the past, allocating precious samples for aggregate testing was completely impractical given the limited availability of material. This is no longer the case.
The Aura GT System is uniquely designed to detect, count, and characterize AAV particles using only 5 µL of sample. By integrating backgrounded membrane imaging (BMI) with fluorescence membrane microscopy (FMM) dual-channel technology, users can make early decisions with confidence, accelerating the path to success.
Analyze small volumes: Particle characterization during the early stages of development is possible using as little as 5 µL of the sample.
Characterize and identify viral vectors: The Aura GT System facilitates the differentiation between capsid and non-capsid aggregates. It also allows for the confirmation of DNA leakage as the primary cause of aggregation, immediately upon setup.
Gain a deeper understanding of gene therapy: The Aura GT System offers insight through its dual capabilities of BMI and FMM. These features differentiate and characterize various serotypes, enabling a more profound understanding of gene therapy aggregation and product characteristics. This is achieved without the need for time-consuming and labor-intensive assays.
Specifications
Source: Waters | Wyatt Technology
| Specifications |
|
| Imaging area |
24.6 mm2 |
| Optics |
4x objective |
| Minimum volume |
5 µL (assay dependent) |
| Resolution |
1.0 pixel/µm |
| Detectable size range |
Range from >1 µm (ECD) to <5 mm (ECD) |
| BMI read time |
1 minute/sample |
| FMM read time |
2 FL channels, 30 seconds/sample |
| Software |
Particle Vue 4.x all-in-one Software suite (image capture and analysis) |
Overview
- Assess AAV, lentivirus, and other viral vectors in relation to crucial applications, including aggregation and stability
- Facilitate direct comparisons among various formulations, AAV serotypes, storage conditions, and batches
- Employing only 5 µL of sample, identify, enumerate, and define AAV particles. Confidently pinpoint capsid and DNA aggregates within the gene therapy sample
- Quantify and identify particles ranging from 1 µm to 5 mm
- Measurements can be completed in under a minute per sample, saving time
The Aura GT system is suitable for viral vector and AAV aggregation and stability investigations during gene therapy advancement.
Easily quantify and identify AAV particles
AAV aggregates are often large and subvisible, complicating the evaluation of their effects on patients. The instability of AAVs further contributes to this issue, as they can aggregate into various shapes and forms.
Aura GT provides a streamlined approach to identifying and characterizing these AAV aggregates. The system enables the characterization of AAV particles with minimal volume requirements and high throughput, offering valuable insights into their stability.
With the Aura GT System, you can characterize your AAV particles with the lowest volume requirements, highest throughput, and gain insight into their stability. Image Credit: Waters | Wyatt Technology
Identify root cause and characterize AAV particles at low volume
Researchers have been looking for an answer to why some AAV particles are unstable for a long time. This investigation has been particularly difficult because older systems cannot analyze small amounts of subvisible particles or check DNA content. The Aura GT System solves this problem with only 5 µL of sample.
Using fluorescent dyes, such as thioflavin T (ThT) and SYBR™ Gold, users can easily find capsid aggregates and DNA in the gene therapy sample using FMM. This helps when choosing the best AAV candidates and understanding why aggregation happens early on in development.
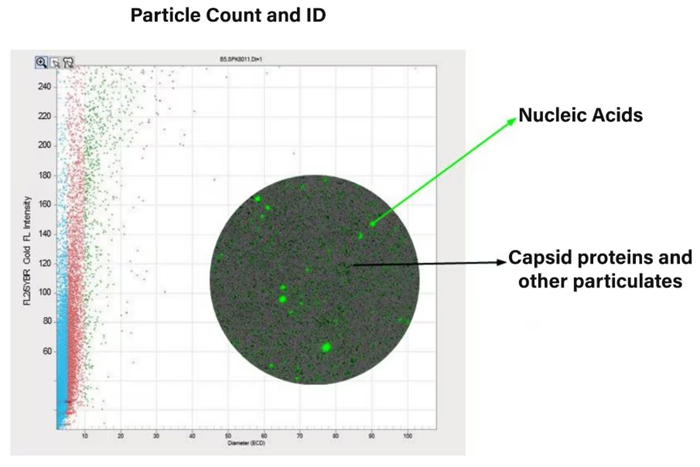
Identify root cause and characterize AAV particles at low volume with the Aura GT System. Image Credit: Waters | Wyatt Technology
Obtain actionable insights in hours, not months
The Aura GT System can help labs expedite the market release of gene therapy products. This system provides insights within hours through its 96-well particle screening assay, significantly reducing the waiting time from months.
Aura GT can compare different formulations directly, evaluating AAV serotypes, stability characteristics, storage parameters, and production batches.

Get your gene therapy products to market even faster with the Aura GT System. Image Credit: Waters | Wyatt Technology