When developing therapies, maintaining protein stability and preventing aggregation is essential. It is now time to move beyond traditional flow cell imaging techniques.
Using Aura PTx, formulation development and protein screening are accelerated. Using only 5 µL of a sample, Aura PTx provides a faster method for detecting, counting and characterizing excipients, and discovering drug products during the initial phases of therapeutic development.
It is now possible to rapidly identify and quantify aggregates resulting from degraded polysorbate within a formulation.
Additionally, with dual-channel fluorescence, Aura PTx can determine whether the aggregation observed in a protein therapy is attributable to proteins or polysorbates present in the sample, and this can be done concurrently.
Specifications
Source: Waters | Wyatt Technology
| Specifications |
|
| Imaging area |
24.6 mm² |
| Optics |
4x objective |
| Minimum volume |
5 µL (assay dependent) |
| Resolution |
1.0 pixel/µm |
| Detectable size range |
>1 µm (ECD) to <5 mm (ECD) |
| BMI read time |
1 minute/sample |
| FMM read time |
2 FL channels, 30 seconds/sample |
| Software |
Particle Vue 4.x all-in-one Software suite |
Overview
- Learn more with innovative technology: The Aura PTx System integrates backgrounded membrane imaging (BMI) alongside dual-channel fluorescence membrane microscopy (FMM). This integration provides comprehensive protein aggregate data, eliminating the necessity for cleaning procedures between successive measurements
- ID protein aggregates: Precisely identify excipient breakdown within formulations and differentiate between protein and non-protein particles with ease
- Monitor polysorbate degradation: The stability of the product can be confidently assessed by tracking polysorbate breakdown using fluorescence applications
- Analyze small volumes: Characterization of protein aggregates can commence earlier in development, as only 5 µL of sample is required
Aura PTx is best suited to the initial identification, quantification, and characterization of protein excipients and drug products during therapeutic development.
Integrated protein stability analysis of biologic formulations
Relying solely on morphological characteristics to determine the optimal strategy is not recommended when dependability, safety, and efficacy are of utmost importance. The Aura PTx System, using FMM, accurately identifies protein (through ThT staining) and non-protein components, even in mixed particles.
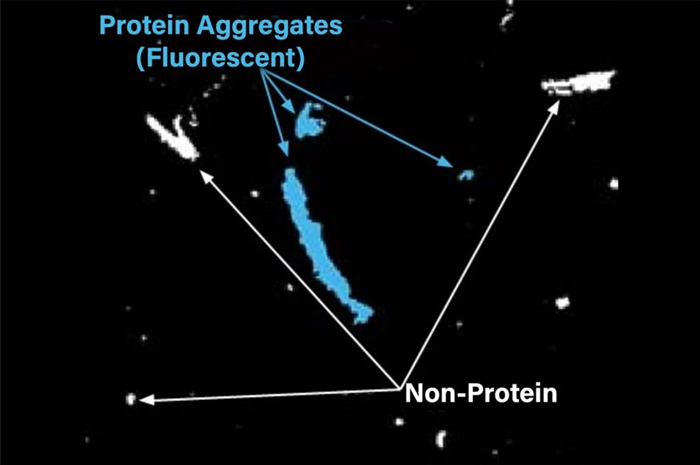
The Aura PTx System tells you exactly what is protein (via ThT staining) and what is not, even in mixed particles. Image Credit: Waters | Wyatt Technology
Identify, count, and size degraded polysorbate
Protein formulations commonly incorporate excipients such as polysorbate to enhance stability. Polysorbate degradation can result in instability.
The Aura PTx System pioneers the use of thioflavin T (ThT) and BODIPY FL C16 staining for the identification and quantification of protein aggregates and polysorbate degradation products. Sample stability concerning both biological aspects and polysorbate content can be assessed in just one experiment.
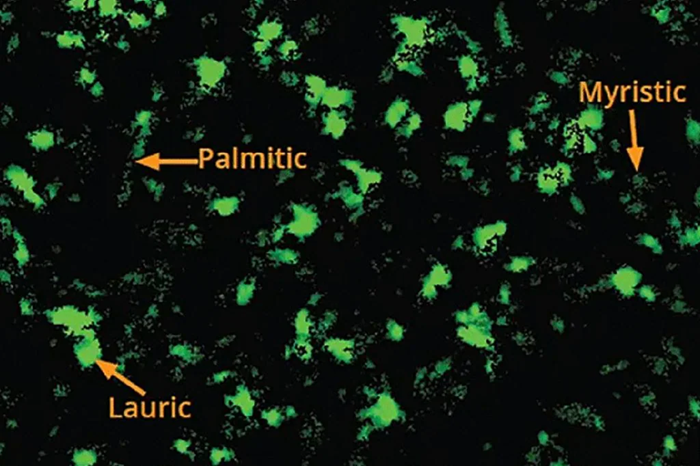
The Aura PTx System is the first to use thioflavin T (ThT) and BODIPY FL C16 staining to detect and quantify protein aggregates and polysorbate breakdown. Image Credit: Waters | Wyatt Technology
High-throughput screening for protein formulations
The Aura PTx System provides a precise method for evaluating the impact of formulations on protein stability, distinct from conventional high-throughput techniques that rely on protein melting point predictions.
The Aura PTx System features an easy-to-use platform that can rapidly examine numerous solutions, completing each analysis in just 60 seconds with minimal stress. Its automated 96-well configuration is well-suited for design-of-experiments (DoE) applications. It requires only a small amount of sample, ensuring confidence in the safety and stability of the drug product throughout its development.

The Aura PTx System incorporates an intuitive platform that can analyze dozens of solutions in just 60 seconds with minimal stress. Image Credit: Waters | Wyatt Technology