Introduction
The evolution of nutritional epigenetics
What is epigenetics?
Biochemical pathways linking maternal nutrition to fetal gene expression
Health outcomes in offspring
Clinical and public health implications
References
Further reading
Nutritional epigenetics examines how maternal diet influences fetal gene expression through mechanisms such as DNA methylation, thereby shaping lifelong risks of metabolic, neurodevelopmental, and immune diseases. This evidence highlights the importance of prenatal nutrition in public health strategies for disease prevention across generations.
 Image Credit: Just Life / Shutterstock.com
Image Credit: Just Life / Shutterstock.com
Introduction
Nutritional epigenetics explains how maternal diet can modulate fetal gene expression without directly altering the DNA sequence and its potential to increase the child’s future risk of developing chronic diseases.1 In public health terms, the critical window spans the periconception period through early gestation, when epigenetic marks are extensively remodeled and may be sensitive to dietary quality and micronutrient availability.3, 4, 5 This article emphasizes clinically actionable insights while retaining mechanistic accuracy.
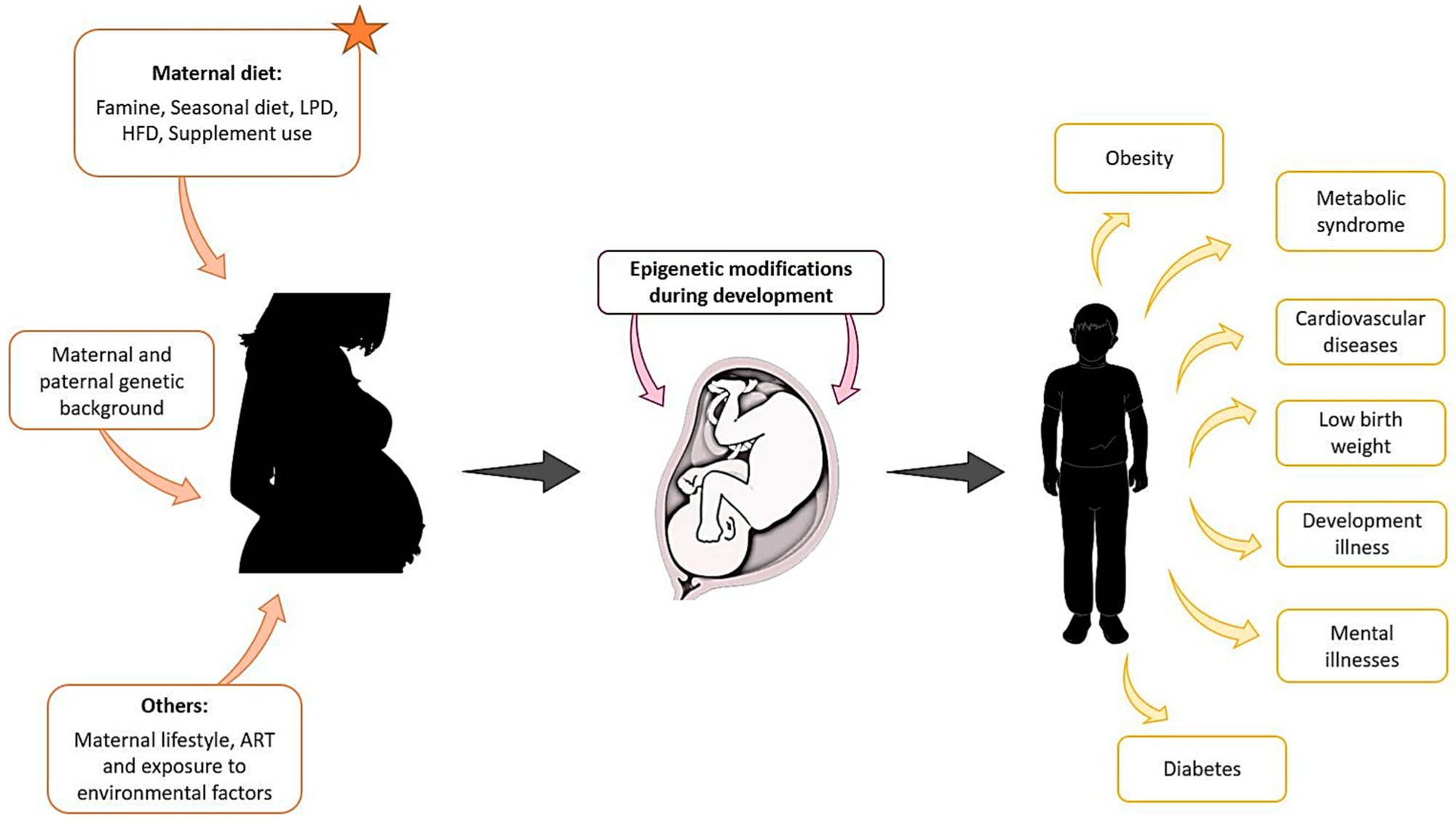
Maternal exposure to environmental factors, diet changes, genetic background, and other parameters such as lifestyle, can affect the development of the foetus from the first stages of pregnancy and compromise the health of the offspring later in life.1
The evolution of nutritional epigenetics
The prevailing view in conventional medicine held that an individual's health was largely predetermined by their DNA sequence. However, more recent research has led to a paradigm shift, revealing that genetic expression is dynamic and profoundly influenced by environmental factors, especially during early development.1
The Barker Hypothesis was the first to establish an epidemiological association between low birth weight and an increased risk of ischemic heart disease in adulthood. Subsequent work consolidated these observations into the DOHaD framework, which proposes that early-life environments shape lifelong disease risk through epigenetic mechanisms that integrate nutritional signals.1, 5
Recent work has expanded upon these hypotheses to form the concept of fetal programming, wherein the fetus makes predictive and adaptive responses to its environment. Thus, adverse health outcomes may arise from a ‘mismatch’ between the environment predicted in utero and that which the infant encounters after birth.1 Clinically, this concept supports preventive strategies that optimize maternal diet before and during pregnancy to reduce later metabolic and cardiovascular risk in offspring.1, 5
What is epigenetics?
Epigenetic modifications are biochemical mechanisms that regulate gene expression without altering the DNA sequence, thereby acting as an interface between the environment and genome.1 Decades of genomic research have revealed three mechanisms through which epigenetic modifications occur, including DNA methylation, histone modification, and non-coding RNAs.1
DNA methylation involves the addition of a methyl group to a preexisting cytosine base, which typically silences the associated gene.1 Mechanistic studies have shown that DNA methylation is a product of the one-carbon cycle, a metabolic pathway that converts dietary nutrients into S-adenosylmethionine (SAM), which subsequently acts as a universal methyl donor.3
The efficiency of this methylation cycle may vary based on maternal intake of folate, vitamin B12, choline, and methionine, thereby biochemically linking nutrition to the epigenetic expressions that regulate the offspring's genome.3 Gene expression can also be controlled by histone modifications like acetylation and methylation that alter the physical accessibility of DNA, whereas non-coding RNAs can directly block the translation of genes into proteins.1 From a clinical perspective, these mechanisms underscore why dietary assessment and micronutrient adequacy matter during the periconception and prenatal periods.3, 4
Biochemical pathways linking maternal nutrition to fetal gene expression
Human studies have demonstrated that maternal periconceptional folic acid supplementation is linked to alterations in offspring DNA methylation at imprinted genes. A key example of this association is that of insulin-like growth factor 2 (IGF2), which regulates fetal growth.4 However, across the placenta and fetal tissues, associations between one-carbon moieties and global DNA methylation are often weak or inconsistent, with more robust, site-specific effects observed in genes involved in growth and stress responses, such as LEP and NR3C1.4
The placenta emerges as a key site where diet-linked methylation changes occur, potentially altering placental function and fetal nutrient signaling. These studies have also revealed that the placenta is a primary site where diet-induced modifications occur, which implies potential alterations to placental function and fetal programming.4
Within one-carbon metabolism, dietary folate, B vitamins, choline, and methionine supply methyl groups to form SAM, yet clinical datasets indicate heterogeneity in how specific nutrients, particularly choline, map to placental methylation outcomes.3, 4
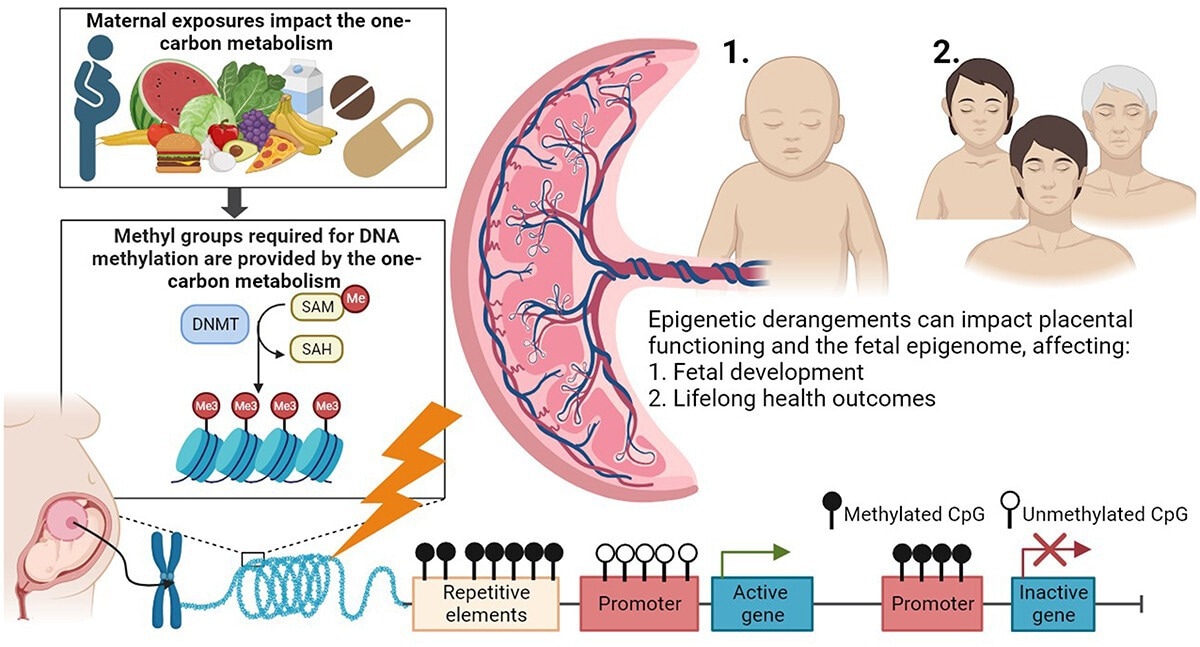
One-carbon metabolism as an underlying pathway affecting placental DNA methylation and lifelong health outcomes in offspring. Maternal exposures, such as nutrition and the use of vitamin supplements, are involved in one-carbon metabolism, which provides methyl groups for DNA methylation processes (left). DNA methyltransferases (DNMTs) transfer methyl groups to the cytosine-phosphate-guanine (CpG) sites. Derangement of the one-carbon metabolism is proposed as a mechanism affecting DNA methylation processes (bottom). The placenta is the intermediate between the maternal environment and embryonic and foetal growth and development. Epigenetic derangements can affect (1) directly the functioning of the placenta and subsequently foetal growth and development, and (2) the foetal epigenome with potential consequences for foetal growth and development, and 1 and 2 both have consequences for lifelong health outcomes in the offspring.4
Animal models of maternal protein restriction support these observations, further demonstrating that this stressor can cause hypomethylation. Maternal malnutrition may also activate histone modifications at the promoters of key metabolic genes, such as the glucocorticoid receptor (GR) and peroxisome proliferator-activated receptor α (PPARα), thereby increasing the future risk of hypertension and metabolic syndrome in these infants. These models inform clinical plausibility but require careful translation to human populations.1, 5
Epigenetic echoes of your mother's diet | Andrew Prentice | TEDxLSHTM
Health outcomes in offspring
In the context of metabolic disorders, maternal undernutrition and overnutrition can epigenetically dysregulate pathways in insulin signaling and lipid metabolism. For example, caloric restriction during pregnancy alters the methylation of genes in adipose tissue, thereby predisposing offspring to metabolic abnormalities during adulthood. Recent large-animal studies in swine have linked gestational caloric restriction with differential methylation of metabolic and inflammatory genes in offspring adipose tissue, alongside adverse metabolic profiles at weaning.1,6,7
Maternal nutritional status may also impact the methylation of genes crucial for neuronal development, such as the brain-derived neurotrophic factor (BDNF) and the glucocorticoid receptor gene (NR3C1). Maternal obesity and HFHS diets have also been associated with an increased risk for neurodevelopmental disorders like autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD). Evidence from rodent and human studies suggests that inflammatory signaling, gut dysbiosis, and epigenetic regulation are convergent mechanisms, although the causal pathways in humans remain under active investigation.8
Maternal dietary habits have a direct impact on the composition of the gut microbiome, which can subsequently influence immune cell maturation through epigenetic mechanisms.9 In 2024, a synthesis of human and experimental data linked maternal Western-style dietary patterns to altered neonatal innate immune programming and pro-inflammatory phenotypes, with plausible epigenetic intermediates.9 These immune shifts may contribute to trajectories of allergy and metabolic disease, warranting preventive dietary guidance and early-life risk stratification.6,9
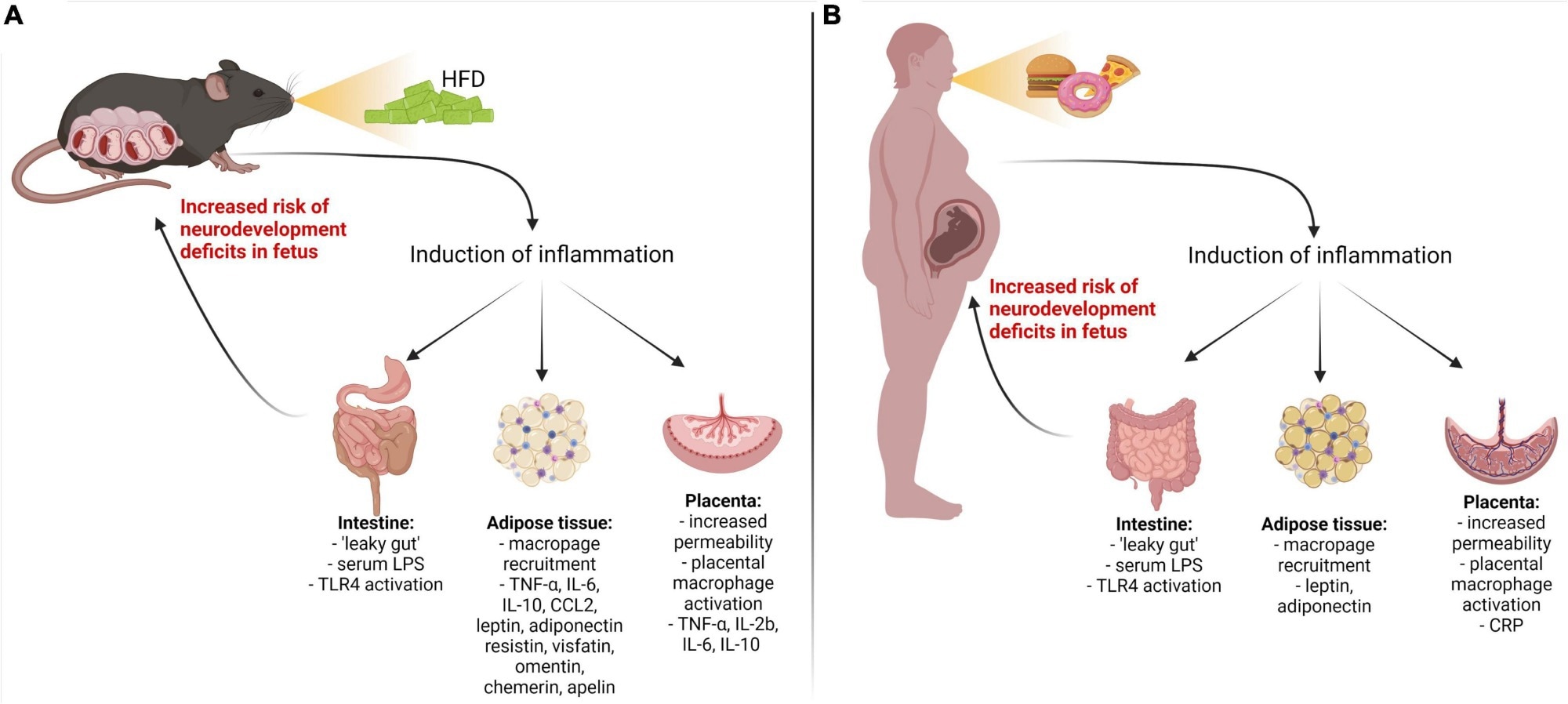
Inflammatory pathways are activated by a maternal high-fat diet-induced metabolic disease. Metabolic disorders in pregnant mouse dams (A) and women (B) result in the upregulation of pro-inflammatory cytokines/adipokines through the gut, adipose, and placental pathways. LPS, lipopolysaccharide; TLR4, Toll-like receptor 4; IL-1β, IL-6, IL-8, IL-17a – interleukins 1 beta, 6, 8, 17a; TNF-α, tumor necrosis factor-alpha; CRP, C-reactive protein; CCL2, chemokine (C–C motif) ligand; IFN-γ, interferon-gamma.8
Clinical and public health implications
Current prenatal dietary guidelines, such as the United States Food and Drug Administration (FDA) recommendation to consume 400 micrograms of folic acid daily to prevent neural tube defects, are considered essential for ensuring optimal fetal outcomes. Public health practice should prioritize preconception and first-trimester folate sufficiency through supplementation and fortified foods, with attention to equity gaps in access and adherence.10
Beyond folate, emphasize balanced dietary patterns that supply one-carbon cofactors and high-quality macronutrients, while avoiding excess saturated fat and free sugars that are associated with maladaptive metabolic and neuroimmune programming in offspring.3, 8, 9 At population scale, recommendations should reflect the current evidence gradient: global methylation endpoints show inconsistency, whereas gene- and tissue-specific effects, particularly in placenta and adipose tissue, have stronger support.4, 7
Risk communication must avoid stigmatization and undue blame. Media narratives around DOHaD can inadvertently individualize responsibility; public health messaging should focus on structural supports for healthy nutrition, including food policy, prenatal services, and culturally appropriate counseling.2
Future clinical translation may include targeted nutritional interventions during the periconception window, integration of maternal diet screening into prenatal care, and judicious use of multi-omics to identify high-risk dyads, recognizing that most mechanistic data remain preclinical.1, 3, 4
References
- Peral-Sanchez, I., Hojeij, B., Ojeda, D. A., et al. (2021). Epigenetics in the Uterine Environment: How Maternal Diet and ART May Influence the Epigenome in the Offspring with Long-Term Health Consequences. Genes 13(1); 31. DOI:10.3390/genes13010031, https://www.mdpi.com/2073-4425/13/1/31.
- Stelmach, A. (2025). Early life, risk and blame: Developmental Origins of Health and Disease (DOHaD) in the news, 1988–2023. Health, Risk & Society 1-17. DOI:10.1080/13698575.2025.2566987, https://www.tandfonline.com/doi/full/10.1080/13698575.2025.2566987.
- Clare, C. E., Brassington, A. H., Kwong, W. Y., & Sinclair, K. D. (2019). One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annual Review of Animal Biosciences 7(1); 263-287. DOI:10.1146/annurev-animal-020518-115206, https://www.annualreviews.org/content/journals/10.1146/annurev-animal-020518-115206.
- van Vliet, M. M., Schoenmakers, S., Gribnau, J., & Steegers-Theunissen, R. P. M. (2024). The one-carbon metabolism as an underlying pathway for placental DNA methylation – a systematic review. Epigenetics 19(1). DOI:10.1080/15592294.2024.2318516, https://www.tandfonline.com/doi/full/10.1080/15592294.2024.2318516.
- Lillycrop, K. A. (2011). Effect of maternal diet on the epigenome: implications for human metabolic disease. Proceedings of the Nutrition Society 70(1); 64-72. DOI:10.1017/S0029665110004027, https://www.cambridge.org/core/journals/proceedings-of-the-nutrition-society/article/effect-of-maternal-diet-on-the-epigenome-implications-for-human-metabolic-disease/0E86A59B2B6A1090290A76EF6569893C.
- Faienza, M. F., Urbano, F., Anaclerio, F., et al. (2024). Exploring Maternal Diet-Epigenetic-Gut Microbiome Crosstalk as an Intervention Strategy to Counter Early Obesity Programming. Current Issues in Molecular Biology 46(5); 4358-4378. DOI:10.3390/cimb46050265, https://www.mdpi.com/1467-3045/46/5/265.
- Mas-Parés, B., Xargay-Torrent, S., Carreras-Badosa, G., et al. (2024). Gestational Caloric Restriction Alters Adipose Tissue Methylome and Offspring's Metabolic Profile in a Swine Model. International Journal of Molecular Sciences 25(2); 1128. DOI:10.3390/ijms25021128, https://www.mdpi.com/1422-0067/25/2/1128.
- Urbonaite, G., Knyzeliene, A., Bunn, F. S., et al. (2022). The impact of maternal high-fat diet on offspring neurodevelopment. Frontiers in Neuroscience 16. DOI:10.3389/fnins.2022.909762, https://www.frontiersin.org/articles/10.3389/fnins.2022.909762/full.
- Nelson, B. N., & Friedman, J. E. (2024). Developmental Programming of the Fetal Immune System by Maternal Western-Style Diet: Mechanisms and Implications for Disease Pathways in the Offspring. International Journal of Molecular Sciences 25(11); 5951. DOI:10.3390/ijms25115951, https://www.mdpi.com/1422-0067/25/11/5951.
- U.S. Food and Drug Administration. (2024). Dietary Advice Before and During Pregnancy. https://www.fda.gov/food/people-risk-foodborne-illness/dietary-advice-and-during-pregnancy. Accessed 24 October 2025.
Further Reading
Last Updated: Nov 9, 2025