Introduction
Taxonomy and ecology
Virulence factors and pathogenic mechanisms
Routes of infection and disease manifestations
Diagnosis and treatment
Epidemiology and risk factors
Public health challenges
Prevention strategies
Conclusions
References
Further reading
Vibrio vulnificus is a life-threatening marine bacterium, increasingly linked to seafood consumption and wound infections, especially in vulnerable people. Its geographic range and public health impact are growing due to climate and environmental change.
 Flesh-eating bacterial infection. Image Credit: Lightspring / Shutterstock.com
Flesh-eating bacterial infection. Image Credit: Lightspring / Shutterstock.com
Introduction
Vibrio species, particularly Vibrio vulnificus, are increasingly recognized as important and sometimes severe public-health hazards capable of causing rapid septicemia, gastroenteritis, and necrotizing wound infections. Climate‑driven proliferation and dietary habits have converged to increase the incidence of vibriosis and related septic shock among high-risk individuals in coastal communities throughout the world.1
Taxonomy and ecology
Vibrio vulnificus is a Gram‑negative halophilic member of the family Vibrionaceae that thrives in warm, low‑salinity estuaries where tidal mixing concentrates organic matter. The curved rods of Vibrio vulnificus are routinely recovered from brackish coastal waters and bio‑accumulated by filter‑feeding shellfish. In particular, the eastern oyster (Crassostrea virginica) acts as both an environmental reservoir and a vehicle for human exposure.
 Eastern Oyster. Crassostrea virginica. Image Credit: Gilbert S. Grant / Shutterstock
Eastern Oyster. Crassostrea virginica. Image Credit: Gilbert S. Grant / Shutterstock
Nutrient‑rich runoff, suspended particulates, and other anthropogenic inputs further contribute to Vibrio vulnificus proliferation by supplying carbon nutrients and attachment surfaces. The organism is rarely detected in waters below 15 °C and is most abundant above 20 °C. Taken together, temperature, salinity, and nutrient availability interact synergistically to modulate the ecology and health risk of this opportunistic pathogen.2
Virulence factors and pathogenic mechanisms
Vibrio vulnificus invades hosts with a multi-layered arsenal. A surface capsular polysaccharide (CPS) masks complement fragments to avoid macrophage engulfment, thereby protecting the bacterium from serum killing.3
Secreted pore‑forming toxins, led by Vibrio vulnificus hemolysin A (VvhA), rupture cell membranes, precipitate dermal necrosis, and induce a cytokine storm that can eventually progress to septic shock. Other virulence determinants include the metalloprotease VvpE, multifunctional RTX toxins, and additional outer-membrane proteins that contribute to tissue invasion and immune evasion. Importantly, systemic dissemination is dependent upon iron levels, as the catechol siderophore vulnibactin and its outer‑membrane receptor obtain iron from transferrin and lactoferrin to ensure bloodstream proliferation.3
In estuarine niches, high levels of cyclic di‑guanosine monophosphate (c‑di‑GMP) and type IV pili contribute to the development of biofilm matrices that shield cells between hosts. Expression of these traits is determined by autoinducer‑2 synthase LuxS and small colony regulator (SmcR) quorum‑sensing circuitry, as mutants lacking either regulator exhibit significantly reduced cytotoxicity and require higher inocula to cause lethal disease.3
Routes of infection and disease manifestations
Vibrio vulnificus infects humans through ingestion or percutaneous exposure. For example, ingestion of raw or undercooked shellfish, especially oysters, deposits high bacterial loads into the gastrointestinal tract.
In most healthy people, this leads to a brief gastroenteritis characterized by diarrhea, cramps, and vomiting. Importantly, Vibrio vulnificus can cross the intestinal barrier to reach the bloodstream, which leads to primary septicemia (PS) with a fatality rate of up to 60%. However, bloodstream infection develops primarily in immunocompromised or chronically ill patients, and the overall incidence of septicemia is low among healthy adults.
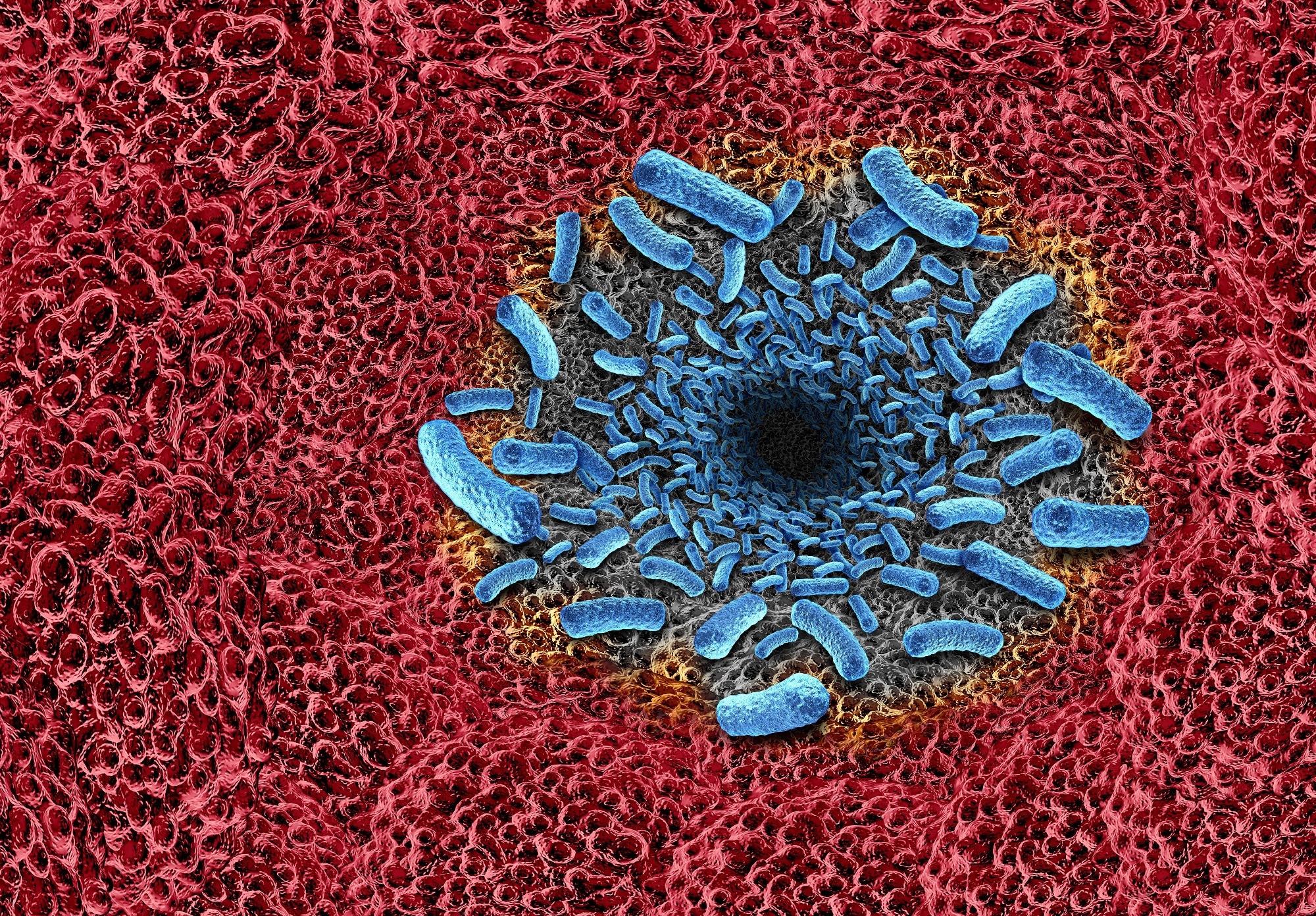
Percutaneous exposure to seawater or seafood contaminated with Vibrio vulnificus can lead to infection by entering the bloodstream through an open wound. Local infection begins with painful swelling and hemorrhagic bullae that can progress within hours to necrotizing fasciitis, sometimes necessitating amputation.4
Patients diagnosed with chronic liver disease, diabetes mellitus, malignancy, renal dysfunction, acquired immunodeficiency syndrome (AIDS), or other forms of immunosuppression are at a significantly greater risk of fulminant PS due to their compromised innate defenses that allow bacterial growth.
Risk of Vibrio vulnificus infection is particularly high during warm summer and autumn when bacterial replication accelerates. As a result, seafood handlers, swimmers, and coastal residents are advised to avoid consuming raw shellfish, promptly disinfect cuts, and seek urgent care for spreading limb pain. Early culture‑guided antibiotics and, when indicated, aggressive surgical debridement remain vital to survival and intensive support.4
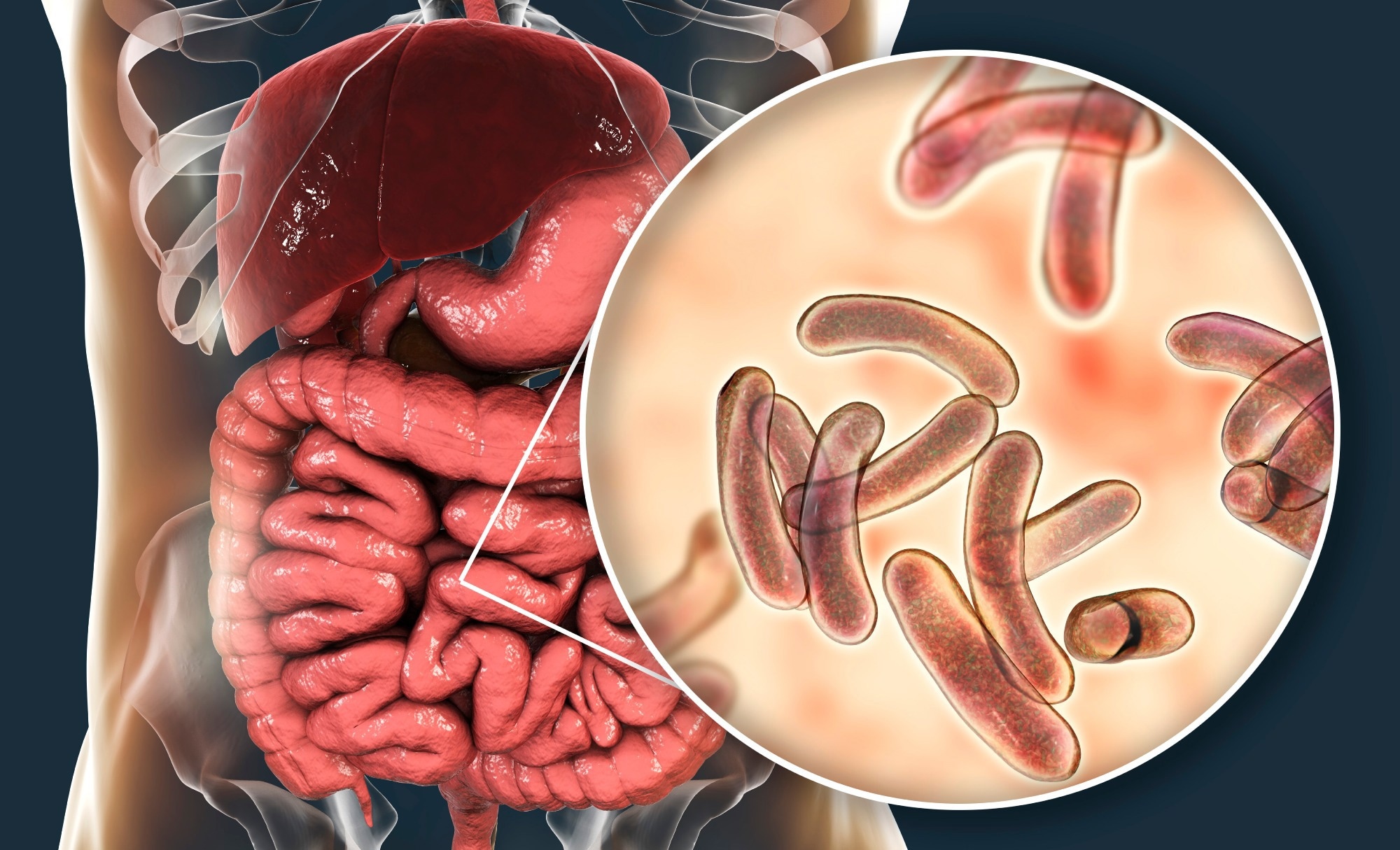 Image Credit: Kateryna Kon / Shutterstock.com
Image Credit: Kateryna Kon / Shutterstock.com
Diagnosis and treatment
Timely recognition of Vibrio vulnificus infection is based on microbiological confirmation, following which immediate and aggressive management is essential. Blood and wound samples, including blister fluid, should be cultured on thiosulfate‑citrate‑bile‑salts‑sucrose agar.
Gram stain of the specimen often reveals banana‑shaped Gram‑negative rods. Since conventional culturing requires up to 48 hours to complete, polymerase chain reaction (PCR) or matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) methods are frequently used to identify the pathogen within hours to accurately guide therapy.
Any broad‑spectrum agent active against Gram‑negative bacilli can be used to treat Vibrio vulnificus. However, doxycycline combined with a third‑generation cephalosporin (such as ceftriaxone or ceftazidime) is widely recommended due to the organism’s rapid doubling time. Fluoroquinolones are an alternative. Imaging is often used to determine a diagnosis of gas gangrene, whereas laboratory tests screen for coagulopathy, hepatic, or renal dysfunction.5
Debridement of devitalized tissue under general anesthesia should begin as soon as feasible after diagnosis. Primary limb amputation is advised if the fascia and muscle are overtly necrotic or the haemodynamic status deteriorates. Throughout resuscitation, clinicians will prioritize circulatory stabilization and support in an intensive care setting to prevent or reverse septic shock and multiorgan failure.5
Epidemiology and risk factors
Vibrio vulnificus thrives in warm, brackish seas and can be found along many temperate‑to‑tropical shorelines. In the United States, these infections have primarily been reported along the Gulf of Mexico; however, Vibrio vulnificus has also been detected along the Atlantic and Pacific coasts, Hawaii, and, occasionally, inland after storm flooding.
In East Asia, especially Japan and China, Vibrio vulnificus prevalence in seafood often exceeds 30%, which is significantly greater than its levels in West Asian waters. Peak reporting occurs between May and October, a period marked by high sea‑surface temperatures of 20-25 °C and high levels of bacterial contamination.6,7
Host factors often determine infection outcomes, as adults with alcoholic cirrhosis are 80-and 200-times more likely to become infected and die after eating raw oysters than healthy diners, respectively. The 80-fold risk refers to infection, and the 200-fold risk to mortality. Other high‑risk groups include people diagnosed with hemochromatosis, thalassemia major, diabetes mellitus, chronic kidney disease, or medication‑induced immunosuppression.6,7
As oceans warm, fisheries and recreational waters deliver Vibrio vulnificus to new latitudes where seafood supply chains and coastal wounds facilitate its circulation. These converging environmental and host factors lead to summertime surges of fulminant septicemia and necrotizing wound infections that have been increasingly reported from the Gulf Coast to East Asia and other warming estuaries throughout the world.6,7
Public health challenges
Surveillance efforts for Vibrio vulnificus are primarily concentrated in the United States and Europe. Comparatively, low‑income countries lack routine testing, which leads to the under‑reporting of cases and weaker risk models.
Climate change is also transporting the bacterium into waters once considered safe. For example, heat‑driven blooms have pushed infections as far north as sub‑Arctic Finland and expanded suitable coastlines by about 392 km² since 1982.
Raw seafood, particularly filter‑feeding oysters, can concentrate Vibrio vulnificus, thereby serving as a potent vehicle for infection. Economically, fulminant septicemia and necrotizing fasciitis often demand prolonged intensive care unit (ICU) management, which makes Vibrio vulnificus one of the most expensive marine‑borne pathogens per case, with an estimated annual burden valued at over $267 million USD.8
New study find cases of flesh eating bacteria may rise
Prevention strategies
Vulnerable patient populations are advised to avoid raw or under‑cooked shellfish altogether to reduce their risk of infection. Cooking shellfish thoroughly to an internal temperature of at least 63 °C (145 °F) effectively kills Vibrio species. Individuals with open wounds, recent piercings, or tattoos must ensure that these areas remain dry and protected while swimming, wading, or handling seafood to prevent bacterial entry.
Federal regulators are expected to perform continuous water‑quality monitoring and impose prompt harvesting closures in the event that water temperature, salinity, or bacterial counts exceed safety limits. Sustained public health campaigns in coastal and endemic areas should teach safe seafood practices, wound care, and early symptom recognition.9 Note: Reference 9 primarily addresses prevention in aquaculture; for human public health, guidance is available from CDC and WHO sources.
Conclusions
Rising sea‑surface temperatures, altered salinity, and nutrient‑rich runoff are expanding the ecological range of Vibrio vulnificus, thereby increasing the risk of infection outside of traditional Gulf Coast and East Asian hotspots.
Protecting public health requires coordinated action across healthcare, environmental monitoring, aquaculture, and food‑safety sectors. These efforts must incorporate routine coastal surveillance, rapid detection technologies, stricter shellfish harvest controls, climate‑informed warning systems, and ongoing community education to ensure the early detection and mitigation of future outbreaks.
References
- Igbinosa, E. O., & Okoh, A. I. (2008). Emerging Vibrio species: an unending threat to public health in developing countries. Research in microbiology. 159(7-8) 495-506. DOI: 10.1016/j.resmic.2008.07.001, https://www.sciencedirect.com/science/article/pii/S0923250808001137
- Quigg, A., Gaona-Hernández, A., Hochman, M. S., Ray, S. M., & Schwarz, J. R. (2025). Applied Time Series Analyses (2000–2017) of Vibrio vulnificus and Vibrio parahaemolyticus (Pathogenic and Non-Pathogenic Strains) in the Eastern Oyster, Crassostrea virginica. Bacteria. 4(2). DOI: 10.3390/bacteria4020017, https://www.mdpi.com/2674-1334/4/2/17
- Jones MK, Oliver JD. (2009). Vibrio vulnificus: Disease and Pathogenesis. Infect Immun. 77 (5). DOI: 10.1128/iai.01046-08, https://journals.asm.org/doi/10.1128/iai.01046-08
- Zu, S., Lin, L., Wen, M. H., Zhao, H. R., Hu, X. M., & Zheng, L. (2024). Secondary septic shock caused by Vibrio vulnificus infection: A case report. LabMed Discovery. 1(2). DOI: 10.1016/j.lmd.2024.100023, https://www.sciencedirect.com/science/article/pii/S3050474024000235
- Matsuoka, Y., Nakayama, Y., Yamada, T., Nakagawachi, A., Matsumoto, K., Nakamura, K., Sugiyama, K., Tanigawa, Y., Kakiuchi, Y. and Sakaguchi, Y. (2013). Accurate diagnosis and treatment of Vibrio vulnificus infection: a retrospective study of 12 cases. Brazilian Journal of Infectious Diseases. 17. 07-12. DOI: 10.1016/j.bjid.2012.07.017, https://www.sciencedirect.com/science/article/pii/S141386701200219X
- Clemence MA, Guerrant RL. 2015. Infections and intoxications from the ocean: risks of the shore. Microbiol Spectrum. 3(6). DOI: 10.1128/microbiolspec.iol5-0008-2015, https://journals.asm.org/doi/10.1128/microbiolspec.iol5-0008-2015
- Tanveer, M., Ntakiyisumba, E., & Won, G. (2024). Prevalence and risk factors of seafood-borne Vibrio vulnificus in Asia: a systematic review with meta-analysis and meta-regression. Frontiers in Microbiology. 15. DOI: 10.3389/fmicb.2024.1363560, https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2024.1363560/full
- Jayakumar, J. M., Martinez-Urtaza, J., Brumfield, K. D., Jutla, A. S., Colwell, R. R., Cordero, O. X., & Almagro-Moreno, S. (2024) Climate change and Vibrio vulnificus dynamics: A blueprint for infectious diseases. PLoS Pathog. 20(12). DOI: 10.1371/journal.ppat.1012767, https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1012767
- Xu, K., Wang, Y., Yang, W., Cai, H., Zhang, Y., & Huang, L. (2023). Strategies for Prevention and Control of Vibriosis in Asian Fish Culture. Vaccines. 11(1). DOI: 10.3390/vaccines11010098
Further Reading
Last Updated: Aug 4, 2025