Jul 7 2009
AbbeyMoor Medical Inc., a developer and manufacturer of medical devices for the management and treatment of prostatic obstruction, announced today that it has received PMA approval from the US Food and Drug Administration (FDA) for design changes to their flagship product, The Spanner Prostatic Stent.
"These changes are aimed at further improving patient comfort over the current highly accepted levels," said Darren Cook, director of marketing with the company. "Our goal is to hear an even greater percentage of our patient's state that they are not aware of the device's presence." According to Mark Whalen, VP of product development with the company, "The approved changes to the device result in a significant increase in flexibility over the current Spanner design with no impact to the ease of delivery and removal of the device." 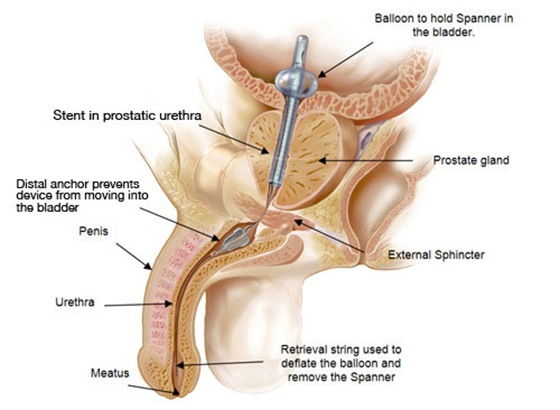
The Spanner is a temporary prostatic stent designed to aid men who are experiencing difficulty in urination due to prostatic obstruction. Device placement can be performed in a physician office and does not require cystoscopy or anesthesia for placement. The Spanner is an alternative to indwelling catheterization, intermittent catheterization, or the use of a suprapubic tube in some patients.
The Spanner temporary prostatic stent has been designed to open the prostatic urethra and allow patients to maintain urine flow and allow volitional voiding by reducing urethral resistance within the prostate. As shown in the figure to the right the Spanner has a proximal balloon which is seated in the bladder neck to properly position the stent in the prostatic urethra. The stent extends from the bladder to just above the external sphincter. Device tethers (suture material) traverse the external sphincter to allow normal sphincter function, thus providing patients the ability to volitionally void. The distal anchor of the device is positioned in the bulbar urethra, just below the sphincter, to prevent device movement and migration into the bladder.