Energy balance between energy intake and expenditure in our bodies is important for maintaining energy homeostasis to keep our bodies functioning properly. The appetite determines how much we eat, the energy intake, by communication between the brain and body.
The dysregulation of the appetite is associated with many diseases. For example, non-balanced dietary habit results in abnormal regulation of appetite, which leads to obesity, one of the main risk factors for diabetes and its complications.
Also, adverse health consequences that stem from eating disorders are more severe than people usually think. Therefore, the studies on appetite control and its mechanisms are of importance in providing therapeutic approaches for the treatment of obesity-related diseases and eating disorders.
How much was previously known about the way the region of the brain called the hypothalamus senses sugar levels in the blood and regulates food uptake?
The hypothalamus maintains energy homeostasis by regulating homeostatic food intake. The specific hypothalamic neurons sense nutritional and hormonal signals from the blood. It has been reported that the hypothalamus increases appetite by sensing low glucose availability, and decreases it under glucose repletion.
What questions currently remain about the mechanisms by which the hypothalamus does this?
Although it has been established that hypothalamic neurons regulate appetite by regulating the expression of appetite-regulating neuropeptide by sensing glucose levels, the precise mechanism remains unknown. Especially how autophagy is involved in feeding control is poorly understood.
Can you please give an overview of your recent research studying the way the brain responds to low glucose availability?
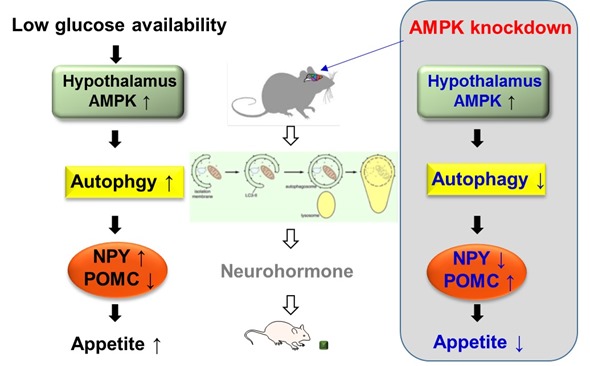
When food is not provided for a certain amount of time, blood glucose levels go down. We proposed the mechanism by which the hypothalamus controls appetite under low glucose availability. Under this condition, in the hypothalamus, AMP-activated protein kinase (AMPK) known as an energy sensor in the cell, senses the drop and gets activated.
Once AMPK is activated, it activates autophagy and then the induced autophagy increases gene expression of appetite-stimulating neuropeptides and decreases that of appetite -inhibiting neuropeptides. As a result, food intake is elevated under low availability of glucose.
What were your main findings?
Recent studies have found numerous functions of autophagy in addition to the removal of unnecessary components. In our study, we discovered an intriguing role of hypothalamic AMPK-autophagy axis involved in feeding control, by specific regulation of feeding-regulatory neuropeptide expression.
Were you surprised by the use of the “self-destruct” mechanism?
As its name suggests (auto: self; phagy: eating), autophagy is originally known as destructive mechanism of the cell that degrades unnecessary or dysfunctional proteins or organelles. When nutrients are limited or deprived in the cells, the autophagy is induced and degrades intracellular materials to produce new proteins and energy.
We came up with the idea that hypothalamic autophagy might respond to low glucose and be involved in signalling to an increase in food intake.
What were the main challenges in this research and how did you overcome them?
The challenge of our research was how we confirm our hypothesis in vivo. This is because living organisms have a multitude of cell populations that are connected and affect each other compared to a single hypothalamic cell line in vitro.
In addition, interaction between the brain and body or their compensatory responses in vivo makes it considerably difficult to have the same results with in vitro experiment.
To do in vivo experiments, we used mouse models and performed genetic targeting of AMPK in specific hypothalamic regions using lentivirus and stereotaxic surgery.
What impact do you think your findings will have?
Obesity has become a worldwide epidemic. Although overeating does not explain all cases of obesity, many evidence have suggested that dysregulation of appetite is one of the biggest contributors to obesity.
Understanding of the role of hypothalamic AMPK-induced autophagy in appetite control might provide a new strategy for design and lead to cures of metabolic diseases such as obesity and diabetes.
What further research is needed to advance our understanding of appetite regulation?
The mechanisms how hypothalamic autophagy regulates neuropeptides expression and what other factors besides low glucose affect hypothalamic autophagy should be further investigated in the future. The ways by which our appetite is regulated are much more complex.
Our appetite can be regulated by not only homeostatic way but also via the reward system and in addictive ways. Therefore, the efforts addressing the neurobiological or behavioural profile will help on our understanding of appetite control as well.
Where can readers find more information?
Our research is published in Autophagy journal (http://www.tandfonline.com/loi/kaup20).
About Professor Kim

Name: Kim, Eun-Kyoung
Address: DGIST, 333, Techno Jungang-daero, Hyeonpung-myeon, Dalseong-gun, Daegu, 42988, KOREA.
Current position: Professor, Department of Brain and Cognitive Sciences, DGIST, KOREA
Director, Neurometabolomics Research Center, DGIST, KOREA
Education
- 1991 B.S. Seoul National University, Department of Microbiology, Seoul, Korea
- 1993 M.S. Seoul National University, Department of Microbiology, Seoul, Korea
- 1999 Ph.D. Seoul National University, Department of Microbiology, Seoul, Korea
Professional experience:
- 1999-2000: Postdoctoral Fellow, National Creative Research Center, SungKyunKwan University, KOREA
- 2000-2006: Postdoctoral Fellow, Department of Neuroscience, Johns Hopkins University School of Medicine, USA
- 2006-2006: Research Associate, Department of Neuroscience, Johns Hopkins University School of Medicine, USA
- 2006-2010: Assistant Professor, Department of Food Science and Human Nutrition, Department of Neurology and Ophthalmology, Michigan State University, USA
- 2010-2016: Associate Professor, Department of Brain and Cognitive Sciences, DGIST, KOREA
- 2013-present: Director, Neurometabolomics Research Center, DGIST, KOREA
- 2016~present: Professor, Department of Brain and Cognitive Sciences, DGIST, KOREA
Society:
- 2002~ : Society for Neuroscience (SfN), regular member
- 2010~ : International Autophagy Conference, regular member
- 2011~ : The Korea Society for Brain and Neural Science (KSBNS), regular member
- 2013~ : Korea Society for Biochemistry and Molecular Biology (KSBMB), regular member
Research Interest:
- Appetite control for prevention/treatment for obesity and diabetes
- Neurometabolomics approach for prediagnostic tools
- Autophagy in the regulation of body homeostasis
- Insulin actions on obesity, diabetes and neurodegeneration
Recent publications (* denotes corresponding author):
1. Oh, T.S., Cho, H., Cho, J.H., Yu, S.W., and Kim, E.-K.*(2016) Hypothalamic AMPK-induced autophagy increases food intake by regulating NPY and POMV expression. Autophagy 12(11) doi: 10.1080/15548627.2016.1215382
2. Oh, T.S., Jeon, Y., Kim, S., and Kim, E.-K.* (2016) AMPK as a regulator of food intake and energy balance. CNS and neurological disorder 15(8) doi: 10.2174/1871527315666160815165806
3. Lee, J.-W., Kim, L.E., Shim, H.-J., Kim, E.-K., Hwang, W.C., Min, D.S., and Yu, S.-W. (2016) A translocator protein 18 kDa ligand, Ro5-4864 inhibits ATP-induced NLRP3 inflammasome activation. Biochemical and Biophysical Research Communications doi: 10.1016/j.bbrc.2016.04.080.
4. Ryu J.R., Hong, C.J., Kim, J.Y., Kim, E.-K., Sun, W., and Yu, S.W. (2016) Control of adult neurogenesis by programmed cell death in the mammalian brain. Molecular Brain 9(43) doi: 10.1186/s13041-016-0224-4
5. Yeo, B. K., Hong, C.J., Chung, K.M., Woo, H., Kim, K., Jung, S., Kim, E.-K., and Yu, S.W. (2016) Valosin-containing protein is a key mediator between autophagic cell death and apoptosis in adult hippocampal neural stem cells following insulin withdrawal. Molecular Brain 9(31) doi:I 10.1186/s13041-016-0212-8
6. Lee, J., Kim, K., Yu, S.-W., and Kim, E.-K.* (2016) Wnt3a upregulates brain-derived insulin by increasing NeuroD1 via Wnt/β-catenin signaling in the hypothalamus. Molecular Brain 9(1) doi:24/10.1186/s13041-016-0207-5
7. Lim, Y., Cho, H., and Kim, E.-K.* (2016) Brain metabolism as a modulator of autophagy in neurodegeneration. Brain Research doi: 10.1016/j.brainres.2016.02.049
8. Shim, H., Park, S., lee, J.-W., Park, H.-J., Baek, S.-H., Kim, E.-K., and Yu, S.-W. (2016) Extracts from Dendropanax morbifera leaves have modulatory effects on neuroinflammation in microglia. American Journal of Chinese Medicine 44(1): 119-32
9. Jeon, Y., Aja, S., Ronnett, G.V., and Kim, E.-K.* (2016) D-chiro-inositol glycan reduces food intake by regulating hypothalamic neuropeptide expression via AKT-FoxO1 pathway. Biochemical and Biophysical Research Communications 470(4): 818-23
10. Park, S., Sadanala, K.C., and Kim, E.-K.* (2015) A metabolomics approach to understanding the metabolic link between obesity and diabetes. Mol. Cells 38(7): 587-596
11. Ha, S., Ryu, H.Y., Chung, K.M., Baek, S.-H., Kim, E.-K., and Yu, S.W. (2015) Regulation of autophagic cell death by glycogen synthase kinase-3beta in adult hippocampal neural stem cells following insulin withdrawal. Molecular Brain 8(30)
12. Chung, K.M., Park, H., Jung, S., Ha, S., Yoo, S., Woo, H., Kim, S.W., Kim, E.-K., Moon, C., and Yu, S.W. (2015) Calpain determines the propensity of adult hippocampal neural stem cells to autophagic cell death following insulin withdrawal. Stem Cells 33(10):3052-64
13. Moon, C., Kim, S.Y, Mammen, A., Cho, B., Kim, E.-K., Park, J.I, and Ronnett, G.V. (2015) Phosphoinositide and Erk signaling pathways mediate activity driven olfactory sensory neuronal survival and stress mitigation. Journal of Neurochemistry 134:486-498
14. Kim, S.Y, Yoo, S.-J., Ronnett, G.V., Kim, E.-K., and Moon, C. (2015) Odorant stimulation promotes survival of rodent olfactory receptor neurons via PI3K/Akt activation and Bcl-2 expression. Mol. Cells 38(6): 535-539
15. Jung, M., Lee, J., Seo, H.-Y., Lim, J.S., and Kim, E.-K.* (2015) Cathepsin inhibition-induced lysosomal dysfunction enhances pancreatic beta-cell apoptosis in high glucose. PLOS one 10(1):e0116972
16. McFadden, J.W., Aja, S., Li, Q., Bandaru, V.V., Kim, E.-K., Haughey N.J., Kuhajda, F.P., and Ronnett, G.V. (2014) Increasing fatty acid oxidation remodels the hypothalamic neurometabolome to mitigate stress and inflammation. PLOS one 9(12): e115642.
17. Jung, H.S., Lim, Y., and Kim, E.-K.* (2014) Therapeutic phytogenic compounds for obesity and diabetes. International Journal of Molecular Sciences 15: 21505-21537
18. Lee, Y., and Kim, E.-K.* (2013) AMP-activated protein kinase as a key molecular link between metabolism and clockwork. Experimental and Molecular Medicine 45:e33
19. Baek, S.-H., Bae, O.N., Kim, E.-K.*, and Yu, S.-W. (2013) Poly(ADP-ribose) polymer induces mitochondrial dysfunction and apoptosis inducing factor release for cell death. Mol. Cells 36: 258-266
20. Kim, E.-K.* (2013) Autophagy impairment by inhibition of cathepsins enhances pancreatic beta-cell apoptosis. J of Korean Diabetes 14: Suppl.2, 24-25
21. Klionsky, D.J. et al. (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8(4): 445-544
22. Zhu, J., Aja, S., Kim, E.-K., Park, M. J., Ramamurthy, S., Jia J., Hu, X., Geng, P., and Ronnett, G.V. (2012) Physiological Oxygen Level is Critical for Modeling Neuronal Metabolism In Vitro. Journal of Neuroscience Research 90: 422-434
23. Han, D., Yang, B., Olson, L.K., Greenstein, A., Baek, S.-H., Claycombe, K.J., Goudreau, J.L., Yu, S.-W., and Kim, E.-K.* (2010) Activation of autophagy by modulation of AMP-activated protein kinase protects pancreatic beta cells from high glucose. Biochemical J. 425(3): 541-51
24. Baek, S.-H., Kim, E.-K., Lee, H., Park, S.K., Lookingland, K. J., Goudreau, J. L., Kim, S. W and Yu, S.-W. (2009) Autophagic cell death in adult neural stem cells. Cell Transplantation 18: 209
25. Baek, S.-H., Kim, E.-K., Goudreau, J.L., Lookingland, K.J., Kim, S.W., and Yu, S.-W. (2009) Insulin withdrawal-induced cell death in adult hippocampal neural stem cells as a model of autophagic cell death. Autophagy 5(2): 277-279
26. Yu, S.-W., Baek, S.-H., Brennan, R.T., Bradley, C.J., Park, S.K., Lee, Y.S., Jun, E.J., Lookingland, K.J., Kim, E.-K., Lee, H., Goudreau, J.L., and Kim, S.W. (2008) Autophagic death of adult hippocampal neural stem cells following insulin withdrawal. Stem Cells 26(10): 2602-2610
27. Kim, E.-K.*, Kleman, A. and Ronnett, G.V. (2007) Fatty acid synthase gene regulation in hypothalamic neurons. Neuroscience Letters 423 (3): 200-204
28. Zhou, W., Han, W.F., Landree, L.E., Thupari, J. N., Pinn, M. L., Bililign, T., Kim, E.-K., Vadlamudi, A., Medghalchi, S.M., Meskini R.E., Ronnett, G.V., Townsend, C.A., and Kuhajda, F. P. (2007) Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Research 67 (7): 2964-2971
29. Zhou, H.R., Kim, H., Kim, E.-K., and Claycombe, K.J. (2007) Obesity-associated mouse adipose stem cell secretion of monocyte chemotactic protein-1 (MCP-1). Am J Physiol Endocrinaol Metab. 293 (5): E1153-1158
30. Ronnett, G.V., Kleman, A., Kim, E.-K., Landree, L.E., and Tu, Y. (2006) Fatty acid metabolism, the CNS and feeding. Obesity Aug;14 Suppl 5:201S-207S
31. Kim, E.-K., Landree, L.E., McCullough, L.D., Kuhajda, F.P., and Ronnett, G.V. (2006) Targeting fatty acid metabolism in the treatments of obesity and disorders of CNS cellular energy balance. Central Nervous System Agents in Medicinal Chemistry 6: 15-25 : equal contribution
32. Kim, E.-K., Landree, L.E., Kuhajda, F.P., and Ronnett, G.V. (2005) Modulating fatty acid metabolism and the AMPK pathway as a target for obesity therapy. Current Medicinal Chemistry-Immunology, Endocrine & Metabolic Agent 5(5): 439-450 : equal contribution
33. Ronnett, G.V., Kim, E.-K., Landree, L.E., Tu, Y. (2005) Fatty acid metabolism as a target for obesity therapy. Physiol Behav 85(1):25-35
34. Tu, Y., Thupari, J. N., Kim, E.-K., Pinn, M.L., Moran, T.H., Ronnett, G.V., Kuhajda, F.P. (2005) C75 alters central and peripheral gene expression to reduce food intake and increase energy expenditure. Endocrinology 146(1): 486-493
35. Kim, E.-K., Miller, I., Aja, S., Landree, L.E., Pinn, M., McFadden, J., Kuhajda, F.P., Moran, T.H., and Ronnett, G.V. (2004) C75, fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J. Biol. Chem 279(19): 19970-19976
36. Thupari, J. N., Kim, E.-K., Moran, T.H., Ronnett, G.V., and Kuhajda, F.P. (2004) Chronic C75 treatment of diet-induced obese mice increases fat oxidation and reduces food intake to reduce adipose mass. Am J Physiol Endocrinaol Metab. 287: E97-104
37. Kim, E.-K., Miller, I., Landree, L.E., Felice, F. B.-R., Brown, P., Tehan, T., Townsend, C.A., Lee, W.A., Moran, T.H., Kuhajda, F.P., and Ronnett, G.V. (2002) Expression of FAS within hypothalamic neurons: A model for decreased food intake after C-75 treatment. AM J Physiol Endocrinaol Metab. 283: E867-879
38. Ko, Y.-G., Kim, E.-K., Kim, T., Park H., Park, H.-S., Choi, E.-J., and Kim, S. (2001) Glutamine-dependent antiapoptotic interaction of human glutaminyl-tRNA synthetase with apoptosis signal-regulating kinase 1. J. Biol. Chem 276 (8): 6030-6036
39. Ko, Y.-G., Kang, Y.-S., Kim, E.-K., Park, S.-G., and Kim, S. (2000) Nucleolar localization of human methionyl-tRNA synthetase and its role in ribosomal RNA synthesis. J. Cell Biol. 149: 567-574
40. Kim, E.-K., Jeong, J.-H., Youn, H.S., Koo, Y.B., and Roe, J.-H. (2000) The terminal protein of a linear mitochondrial plasmid is encoded in the N-terminus of the DNA polymerase gene in white-rot fungus Pleurotus ostreatus. Curr Genet 38: 283-290
41. Kim, E.-K., and Roe, J.-H. (1999) Function of mORF1 protein as a terminal recognition factor for the linear mitochondrial plasmid pMLP1 from Pleurotus ostreatus. J. Microbiol. 37: 229-233
42. Kim, E.-K., Youn, H.S., Koo, Y.B., and Roe, J.-H. (1997) The genetic organization of the linear mitochondrial plasmid mlp1 from Pleurotus ostreatus NFFA2. J. Microbiol. 35 (4): 264-270
43. Jeong, J.-H., Kim, E-K., and Roe, J.-H. (1995) Phylogenetic analysis of Pleurotus species based on the nuclear SSU rRNA sequences. J. Microbiol. 34 (1): 37-39
44. Kim, E-K., Koo, Y.B., Cha, D.-Y., Hah, Y. C., and Roe, J.-H. (1993) Characterization of mitochondrial plasmids from Pleurotus ostreatus. Kor. J. Microbiol. 31 (2): 141-147