May 17 2018
Researchers affiliated with the Kawasaki INnovation Gateway at SKYFRONT have developed new mouse models of retinal degeneration that enable transplantation of retinal sheets derived from human embryonic stem cells. These results are reported in the journal Stem Cell Reports and show their approach to be promising for the treatment of retina degeneration by transplanting retinal tissue derived from embryonic or induced pluripotent stem cells.
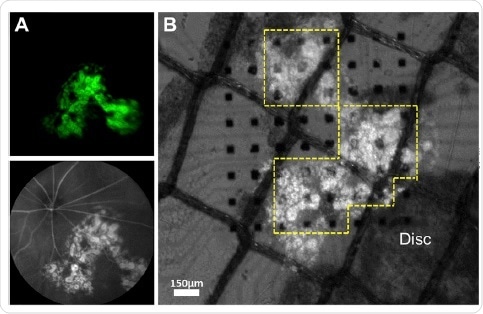
Classic Photoreceptor Responses Elicited in NOG-rd1-2J Mouse after Transplantation of hESC-Derived Retina (A) In vivo imaging of a transplanted eye at postoperative day 112 (DD176). Fluorescent angiography shows the Crx::venus-positive graft under the retinal vessels. No sign of rejection was observed. (B) Isolated retina from (A) with Crx::venus hESC-derived graft (DD204) mounted on the MEA probe with RGC on the electrodes side.
Retinal degeneration, a pathology in which the cells in the retina progressively die, leads to impaired vision and, in some cases, blindness. A promising therapeutic option for the treatment of retina degeneration consists of transplanting retinal tissue derived from embryonic or induced pluripotent stem cells (ES/IPS cells).
It is known that photoreceptor precursor cells derived from embryonic stem cells can be transplanted in adult mouse models of retinal degeneration that still have dysfunctional photoreceptor layer (or outer nuclear layer). In these models, the transplanted photoreceptor cells restored some vision, but it was recently considered due to transfer of materials from the transplanted cells to remaining dysfunctional cells. On the other hand, it has also been shown that the transplanted retinal sheets differentiated from mouse ES or iPS cells can create new synaptic connections and partially restore vision even in models of end-stage retinal degeneration in which there is no functioning photoreceptor layer. However, transplanting retina sheets derived from human embryonic stem cells in rodent models is challenging and often results in rejection and in the transplanted cells not maturing properly.
Thus, immunodeficient end-stage retinal degeneration mouse models without any outer nuclear layer structure are important for preclinical studies investigating the feasibility of transplantation of tissues derived from human embryonic stem cells or induced pluripotent stem cells. In a study just published in Stem Cell Reports by Motohito Goto, Central Institute for Experimental Animals, Animal Resources and Technical Research Center, Kawasaki, Japan, and colleagues, two mouse models developed using marker-assisted breeding are presented and characterized. In the two models, the photoreceptor degeneration proceeds at different speed.
The researchers transplanted retinal sheets derived from human embryonic stem cells in one of the models, characterized the structure of the transplanted retina and of the host-graft interface and measured the response to light. The retinal sheets were integrated for a long period of time, and showed signs of photoreceptor maturation, including the formation of light-responsive inner/outer segments and of synapses. A full-field electroretinography response was not detected (this measures the electrical responses of various cell types in the retina, including the photoreceptors, inner retinal cells, and the ganglion cells), but this does not necessarily imply total blindness. By contrast, retinal ganglion cell responses to light stimuli were observed in 3 transplanted retinas out of 7 that were measured. Thus, there was some indication of a functional integration of the transplanted tissues with the host retina, even though less successful than with mouse-derived stem cells in these mice models.
As the authors conclude:
this study supports the use of these animals in preclinical studies, as well as the competency of human embryonic stem cell retina for future clinical applications in retinitis pigmentosa patients.