Beckman Coulter Life Sciences has published a flow cytometry white paper to support the gating strategies behind its newly launched IVD ClearLLab Control Cells - the first application-specific control for leukemia and lymphoma immunophenotyping in the clinical laboratory.
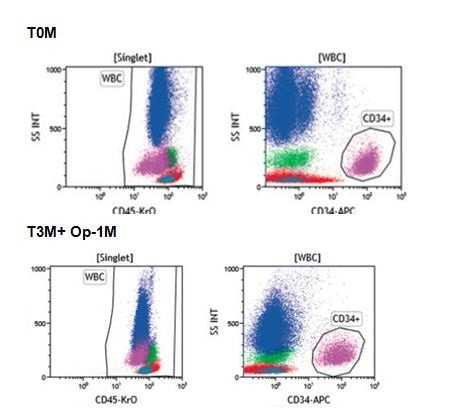
Figure 1: Snapshot of TOM and T3M+ Op-1Mplots. Image credit: Beckman Coulter
The control cells have been designed specifically to verify the performance of the ClearLLab10C panels of reagents and staining procedure, cleared earlier this year by the Food and Drug Administration (FDA) for both lymphoid and myeloid lineages.
Flow cytometric immunophenotyping is increasingly used to aid in the diagnosis of hematolymphoid malignancies. The freely available ClearLLab Control Cells white paper details the flow cytometry gating strategy for B, T, M1 or M2 tubes.
Controls are key for all assays, but until now most labs have had to design their own solutions. ClearLLab Control Cells offer the ideal combination of convenience and consistency, and permit flow cytometry laboratories to streamline this aspect of testing.”
Jeannine Holden MD, Chief Medical Officer, Beckman Coulter
The ClearLLab gating strategy provides the lab with an easy, simple and consistent assessment of every relevant cell population. With the ClearLLab 10C System, labs now have access to a portfolio of standardized, diagnostic tools, freeing up time previously spent on extensive manual validation, preparation and QC tasks.”
Dr. Mario Koksch, Flow Cytometry Business Unit Vice President and GeneralManager, Beckman Coulter Life Sciences
ClearLLab Control Cells are available in both normal and abnormal controls - the only difference is that the abnormal control has a blast-like population that is CD45dim, and expresses CD34, CD123 and CD117. They include assay values for the 27 markers currently available on the four ClearLLab10C panels. These markers are consistent with WHO and Bethesda guidelines.
The control cells are liquid preparations of stabilized human erythrocytes and leukocytes (lymphocytes, monocytes, and granulocytes) that have lysing, light scatter, antigen expression, and antibody staining properties representative of those found in human whole blood specimens.
Erythrocytes function as the lysable component, with leukocytes forming the positive cell component.The surface antigens present on the targeted cells bind to the antibodies in the ClearLLab 10C panels, and include CD34, CD117 and CD123.Multiple lots of both fresh (*T0M) and aged (*T3M+Op-1M) control cells were stained with each of the four ClearLLab 10C panels to assess the staining signal, resolution of each antigen as well as specimen stability.
Figure 1 looks at two markers, Krome Orange (CD45-KrO) and APC (CD34-APC), comparing the plots generated by fresh and aged analysis. The full analysis is given in the white paper.
The fresh (T0M) control cell specimen was tested within 2 weeks of the post-production date. The aged (T3M+ Op-1M) control cell was tested 3-month close-vial plus a 1-month open-vial post production date.
The ClearLLab 10CSystem incorporates the company’s new Kaluza C software to streamline and standardize clinical QC reporting to international guidelines. The four dry pre-mixed antibody tubes also use the company’s DURA Innovations technology, eliminating the need to manually pipette the antibodies.
“The use of dry, preformulated reagents with the ClearLLab 10C System decreases the potential for human error, further improving overall lab efficiency,” concluded Dr. Koksch