As COVID-19 continues to take a heavy toll on human health and life in many parts of the world, there is still no effective antiviral drug or vaccine. This has fueled an intensive search for small molecules and proteins that can inhibit viral entry and replication.
Now, a new study by researchers in the U.S. and published on the preprint server bioRxiv* in August 2020 reports a new orally used compound called PTC299, which is a potent inhibitor of dihydroorotate dehydrogenase (DHODH).
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a single-stranded RNA virus which enters the human host cell via its attachment to the human angiotensin-converting enzyme 2 (ACE2), a membrane protein and an enzyme in the renin-angiotensin pathway. This virus binds to the S1 subunit of the enzyme and undergoes endocytosis to internalize into the host cell. This is followed by a host protease-induced cleavage at the S1/S2 interface, which allows the post-fusion virus to escape from the endosome and enter the cytoplasm.
The virus then replicates rapidly, using the host cell machinery, which sometimes triggers a cytokine storm, the unregulated production and release of inflammatory cytokines. This leads to increased permeability of the blood vessels, multi-organ dysfunction, and acute respiratory distress syndrome (ARDS), and death. The illness is also associated with high levels of IL-6 and IL-17, inflammatory cytokines that are linked to the severity and fatal outcomes of the lung injury observed in these cases.
The researchers attempted to find a compound that can both prevent the replication of the virus while also reducing the cytokine levels, thus proving its usefulness in both early and late COVID-19. They looked at inhibitors of the cellular pathway for de novo biosynthesis of pyrimidines, one of the types of nucleotides that make up the RNA strand. Adequate pyrimidine levels are necessary for both the RNA synthesis required for RNA replication, as well as for RNA transcription that underlies the unregulated release of these cytokines.
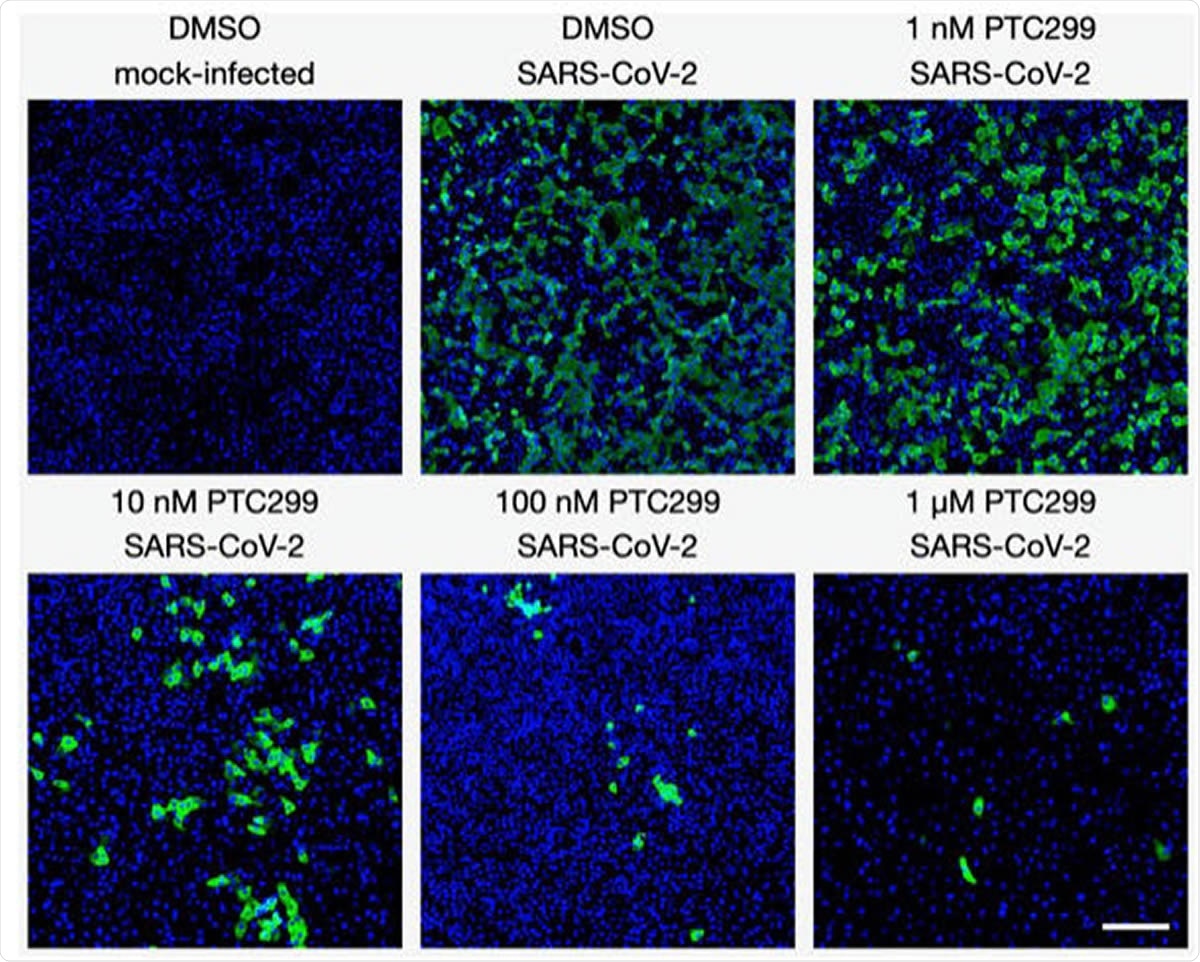
PTC299 inhibits SARS-CoV-2 replication. Quantitative immunofluorescence analysis of SARS-CoV-2-infected Vero E6 cells treated with PTC299. PTC299 was added at concentrations ranging from 1 nM to 1 μM 30 minutes prior to infection of Vero E6 cells with SARS-CoV-2 (USA-WA1/2020) at a multiplicity of infection (MOI) of 0.1. At 48 hours post-infection, the cells were fixed, probed with antibodies against the nucleocapsid protein (NP) of SARS-CoV-2 and stained with AlexaFluor 488 conjugated secondary antibody. Nuclei were stained with DAPI. Images acquired in the Green (i.e., NP of SARSCoV- 2) and the Blue (DAPI) channels were overlaid and are displayed as indicated (note images corresponding to the 3 nM concentration of PTC299 were omitted for simplicity). Scale bar, 200 μm.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
What is DHODH?
DHODH is an enzyme that in humans is encoded by the DHODH gene on chromosome 16. DHODH is the rate-limiting enzyme in this pyrimidine biosynthesis pathway, which means it controls the level of pyrimidines. The protein encoded by this gene catalyzes the fourth enzymatic step, the ubiquinone-mediated oxidation of dihydroorotate to orotate in de novo pyrimidine biosynthesis. This protein is a mitochondrial protein located on the outer surface of the inner mitochondrial membrane (IMM), it metabolizes DHODH to the triphosphates of the pyrimidines uridine and cytidine, UTP, and CTP, respectively. Inhibitors of this enzyme are used to treat autoimmune diseases such as rheumatoid arthritis.
What is PTC299?
The molecule PTC299 is a top contender among the molecules identified by a recent multi-omics study to have a high potential for SARS-CoV-2 treatment. This molecule is a potent inhibitor of this enzyme. Hence, its efficacy in inhibiting viral replication and inflammation would be expected given the central role played by DHODH in infection by this virus.
In vitro suppression of DHODH activity is seen in cells treated with this molecule. It is also seen in cancer patients, where the blood levels of DHO went up on the administration of PTC299 because it inhibited DHODH. High DHO levels are also a feature of Miller syndrome patients, who have an unfavorable mutation in the DHODH gene.
Vascular endothelial growth factor (VEGF) is a cytokine that fluctuates with stress levels. Prior studies also show that PTC299 suppresses VEGF levels both in culture and in cancer patients, who have higher levels.
PTC299 is typically well-tolerated among humans. Thus, all these factors project its suitability as a therapeutic drug for COVID-19. The current study attempts to examine the antiviral activity of this molecule against several RNA viruses and especially SARS-CoV-2, and its inhibitory activity against inflammatory cytokines.
The researchers found that PTC299 reduces the levels of the nucleocapsid protein in a dose-dependent manner, the EC50 being 1.96 nM. However, the number of cells in culture did not go down, showing that the molecule inhibits viral replication without exerting cytotoxic effects.
Secondly, when this drug was added to cells prior to, at or after viral infection, the viral replication dropped sharply. In the first case, the reduction was by about 3 orders after 24 hours. The EC50 was found to be 2.6 nM while the IC50 was 53 nM, The molecule was also found to have a high selectivity index, with the cytotoxic concentration CC50 being over 10 μM, much higher than the IC50.
Broad Spectrum
The results also show that it inhibits all the RNA viruses tested, including potent suppression of HCV, poliovirus, Ebola, and Rift Valley fever virus, with minimal cytotoxicity. The CC50s were, in most cases, above the highest dose of PTC299 used.
Anti-Inflammatory Action
This molecule also inhibits the production of inflammatory cytokines in response to viral infection. In a coculture of peripheral blood mononuclear cells (PBMCs), B cells, fibroblasts and endothelial cells, among others, stimulated to replicate physiological signaling pathways, the addition of PTC299 suppressed several immunomodulatory and inflammatory cytokines which are associated with a poor prognosis in COVID-19. Specifically, PTC299 reduced the levels of MCP-1, CD40, and IL-8, by 27% to 31% at nanomolar concentrations. Endothelial cell proliferation was also reduced by about a fourth after PTC299 treatment.
Chronic inflammatory milieus treated with PTC299 showed the same results, reducing the levels of soluble IgGs by about 50% to 70%, and with an almost complete suppression after 144 hours of stimulation.
Implications for COVID-19 Treatment
The researchers say, “Our results indicate that that PTC299 has considerable potential as a COVID-19 therapeutic.” The cytokine response is a significant contributor to the life-threatening course of the infection in some cases, and inhibitors of inflammation such as the IL-6 inhibitor tocilzumab have shown the potential to modulate the severity of illness. The current study shows that PTC299 can also reduce or suppress deleterious cytokine production, such as MCP-1, IL-6, TNFα, VEGF, and IL-17. It can also reduce the levels of IgG. These could have significant and clinically useful implications in the treatment of COVID-19.
The molecule PTC499, therefore, shows two mechanisms of benefit in the treatment of COVID-19, making it different from most other therapeutic molecules under investigation currently. This property also means it is useful for both early and late disease in COVID-19. In contrast, direct-acting antivirals are most effective when started early.
Secondly, the unique mechanism of action means that viruses are unlikely to develop resistance since they cannot forego the need for pyrimidines in RNA synthesis. And finally, it is a potent inhibitor of viral replication with a nanomolar EC50.
An effective drug will also have a safe dose, which can continue to remain at effective levels in the blood over a long period. A phase 2/3 trial for PTC299, FITE19, is going on at present, using a dose that will yield an average concentration 55 times the EC50 value against SARS-CoV-2. Thus, this molecule shows great promise, combining both antiviral and immunosuppressive activity to clear the virus and also modulate the cytokine storm. Its oral bioavailability, safety profile over extensive human testing, and tolerability add to its clinical utility, making it a front-runner for further development.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources