In a promising study by US researchers, the receptor-binding domain (RBD) and stabilized spike glycoprotein of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were displayed on nanoparticles as vaccine antigens and elicited potent immune responses. The paper is currently freely available on the bioRxiv* preprint server.
The whole world is currently united in the quest for an effective therapeutic and prophylactic option against SARS-CoV-2, a causative agent of coronavirus disease (COVID-19). One of the hurdles is that the underlying immunology of COVID-19 is yet to be fully understood.
The so-called Operation Warp Speed aims to deliver approximately 300 million doses of safe and effective vaccines by January 2021 with the utilization of public, private partnerships, already resulting in five vaccine candidates (in addition to others currently being tested in human trials).
Nonetheless, vaccine development in the midst of a pandemic against a novel virus poses rather unique challenges, one of which is how to reconcile public health needs with obligatory scientific rigor.
Still, such a global quest for vaccine also enables a unique occasion to compare different vaccine designs, approaches, strategies, and platforms against a common target – especially those that represent ingenious technological designs.
In this study, a research group from the United States (led by Dr. Linling He from the Scripps Research Department of Integrative Structural and Computational Biology) designed and optimized SARS-CoV-2 RBD/spike antigens and displayed them on self-assembling protein nanoparticles (SApNPs) as COVID-19 vaccine candidates.
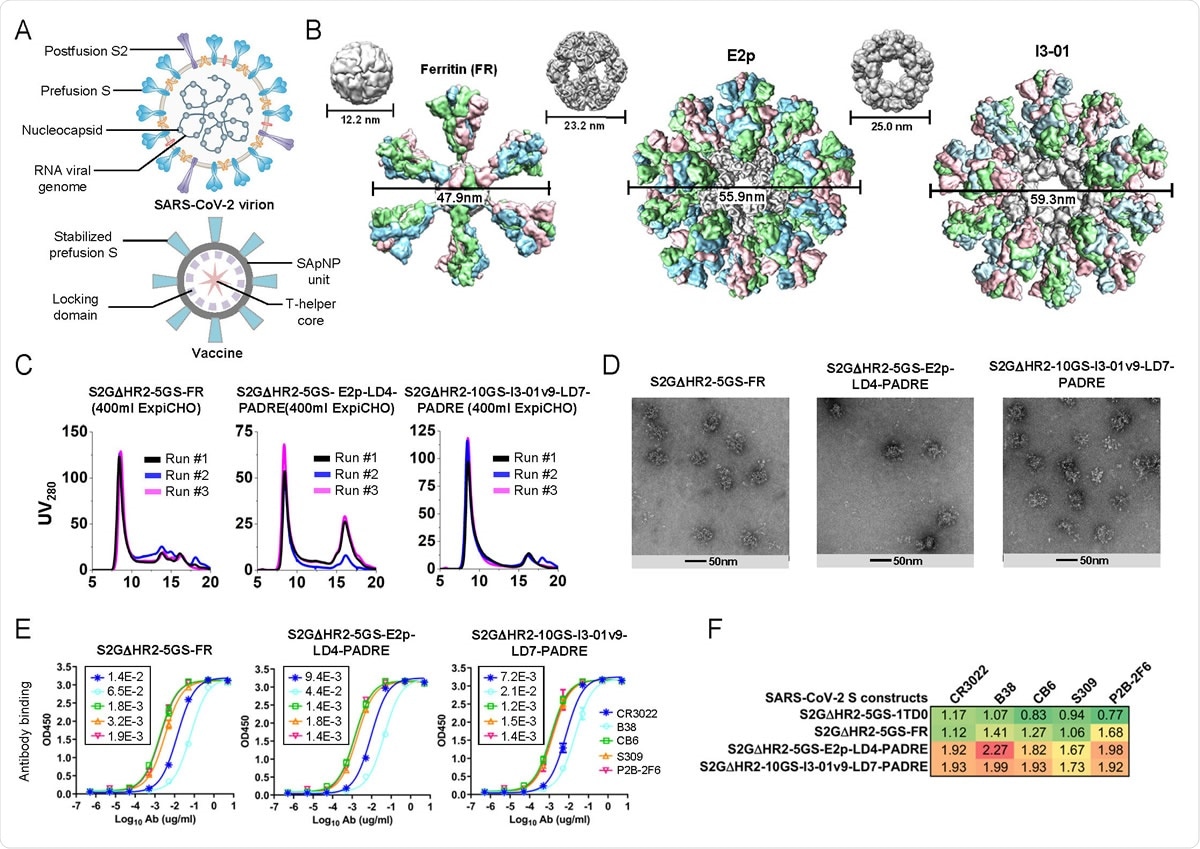
Rational design of SARS-CoV-2 spike-presenting SApNP vaccines. (A) Schematic representation of SARS-CoV-2 virion (top) and spike-presenting SApNP vaccine (bottom). For the SARS-CoV-2 virion, pre/post-fusion S, nucleocapsid and RNA viral genome are labeled, while for the vaccine, stabilized spike and multilayered SApNP carrier are labeled. (B) Colored surface models of SApNP carriers (top) and spike-presenting SApNP vaccines (bottom). Three SApNP carriers shown here are 24-meric ferritin (FR) and 60-meric E2p and I3-01v9. SApNP size is indicated by diameter (in nanometers). (C) SEC profiles of SARS-CoV-2 S2GΔHR2 SApNPs obtained from a Superose 6 10/300 GL column for three separate production runs. (D) EM images of three SARS-CoV-2 spike SApNPs: S2GΔHR2-5GS-FR (left), S2GΔHR2-5GS-E2p-LD4- PADRE (or -L4P, middle), and S2GΔHR2-10GS-I3-01v9-LD7-PADRE (or -L7P, right). (E) ELISA binding of three SARS-CoV-2 spike SApNPs to five mAbs/NAbs. EC50 (μg/ml) values are labeled on the plot. (F) Antigenic profiles of SARS-CoV-2 S2GΔHR2 spike and three SApNPs against five mAbs/NAbs Sensorgrams were obtained from an Octet RED96 using six antigen concentrations (150-4.6nM for the spike, 9-0.27nM for the FR SApNP, and 3.5-0.1nM for the E2p and I3-01v9 SApNPs, respectively, all by twofold dilution) and quantitation biosensors, as shown in fig. S3B. The peak signals (nm) at the highest concentration are listed in the matrix. Color coding indicates the signal strength measured by Octet (green to red: low to high).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
A trifecta of yield, antigenicity and purity
This research group initially developed an RBD-specific antibody column for purification. It displayed the RBD on SApNPs by using the SpyTag/SpyCatcher system (i.e., a technology for irreversible conjugation of recombinant proteins). Of note, RBD represents a key element of SARS-CoV-2 that allows viral binding to the human cells.
Furthermore, to speed up vaccine purification, the researchers have developed an immunoaffinity column based on specific antibody CR3022 that binds to both SARS-CoV-1/2 RBDs. They have also designed a scaffolded RBD trimer to mimic the "RBD-up" spike conformation.
In their subsequent imaginative move, the researchers identified the heptad repeat 2 (HR2) stalk as a significant cause of spike metastability. This prompted the design of an HR2-deleted glycine-capped spike (S2G∆HR2), which resulted in displaying S2G∆HR2 on three SApNPs with high yield, antigenicity, and purity.
Two uncleaved spike antigens, S2P (K986P/V987P) and S2G (K986G/V987G) were compared to probe the spike metastability. Consequently, the SARS-CoV-2 S2G spike exhibited abnormal behavior, implying that an unidentified facet of the spike may promote conformational change and obstruct antibody access to the RBD.
High neutralizing antibody titers
In a nutshell, this study has shown that single-component SApNPs may indeed be a feasible path towards a new and powerful platform for vaccine development against SARS-CoV-2 and other viral pathogens.
The RBD-ferritin SApNP gave rise to a more potent neutralizing antibody response in the mouse study (on par with the spike) when compared to the RBD. Moreover, S2G∆HR2 elicited two-fold-higher neutralizing antibody titers than the S2P, while S2G∆HR2 SApNPs gave rise to 10-fold higher neutralizing antibody titers.
Additional analysis indicated that the S2G∆HR2 could induce a very robust Th1 immune response, as well as other types of T-cell responses that are necessary for mounting protective cellular immunity.
Regarding the latter, it has to be emphasized that emerging evidence points towards T-cell response as crucial when SARS-CoV-2 is concerned. While antibodies are needed to hamper host-virus interaction and, in turn, prevent viral infection, cellular immunity is pivotal for eliminating infected host cells in order to control viral infection fully.
Endorsing a rational design strategy
"In summary, our study provides promising next-generation COVID-19 vaccine candidates that are ready for evaluation in human trials", say study authors in this tour-de-force bioRxiv preprint paper.
Such recombinant protein vaccines should be more effective in inducing a potent anti-SARS-CoV-2 neutralizing antibody response with a lower probability of adverse responses, which is actually a promise this new approach holds.
In any case, the choice of antigen is pivotal for the success of a vaccine, irrespective of the delivery platform. A rational design strategy used for vaccine development in this study is undoubtedly a way forward.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
He, L. et al. (2020). Self-assembling nanoparticles presenting receptor binding domain and stabilized spike as next-generation COVID-19 vaccines. bioRxiv. https://doi.org/10.1101/2020.09.14.296715.
- Peer reviewed and published scientific report.
He, Linling, Xiaohe Lin, Ying Wang, Ciril Abraham, Cindy Sou, Timothy Ngo, Yi Zhang, Ian A. Wilson, and Jiang Zhu. 2021. “Single-Component, Self-Assembling, Protein Nanoparticles Presenting the Receptor Binding Domain and Stabilized Spike as SARS-CoV-2 Vaccine Candidates.” Science Advances 7 (12): eabf1591. https://doi.org/10.1126/sciadv.abf1591. https://www.science.org/doi/10.1126/sciadv.abf1591.