The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the pathogen responsible for the ongoing coronavirus disease 2019 (COVID-19) pandemic – affects the respiratory tract in humans. It is commonly transmitted by close contact with infected persons via airborne particles and aerosols.
The spike protein (S-protein) of the virus infects human host cells by binding to the angiotensin-converting enzyme 2 (ACE2). In-situ RNA mapping has revealed that the expression of ACE2 is highest in the nose, decreasing as one goes down the upper respiratory tract. Similarly, the SARS-CoV-2 infection is higher in upper pulmonary epithelial cultures compared to that in the lower regions. Thus, the first symptoms of infection often are runny nose, congestion and a sore throat.
Although several strategies like social distancing, wearing masks, quarantines, and intensive testing have been implemented almost worldwide, these have met with limited success in many places. Other strategies, like vaccines and therapies, are still being developed. These will require time to develop and deploy.
In the interim, a possible strategy could be to block entry of the virus in the upper respiratory tract. This could also be used in conjunction with vaccination or other antiviral therapies.
Nafamostat mesylate (NM) is an anticoagulant that has been approved for use in Japan. It has also been shown to be a potent inhibitor of spike protein-mediated membrane fusion and an inhibitor of SARS-CoV-2's entry into human lung epithelial cells in vitro.
Testing hamsters with intranasal application of nafamostat mesylate
In a new study on Syrian hamsters, researchers based in Germany and the Netherlands have practice tested the effectiveness of NM in reducing nasal viral loads and COVID-19 severity. The researchers also tested to see if adding NM in a liposome layer could both increase the stability of NM in the nose and act as a mechanical barrier to virus infection.
The authors applied NM intranasally to female Syrian hamsters and, after 5 minutes, inoculated them with SARS-CoV-2 – also applied via the nose. They analyzed the viral loads in the animals from nasal swaps, which were collected 1 and 3 days after infection.
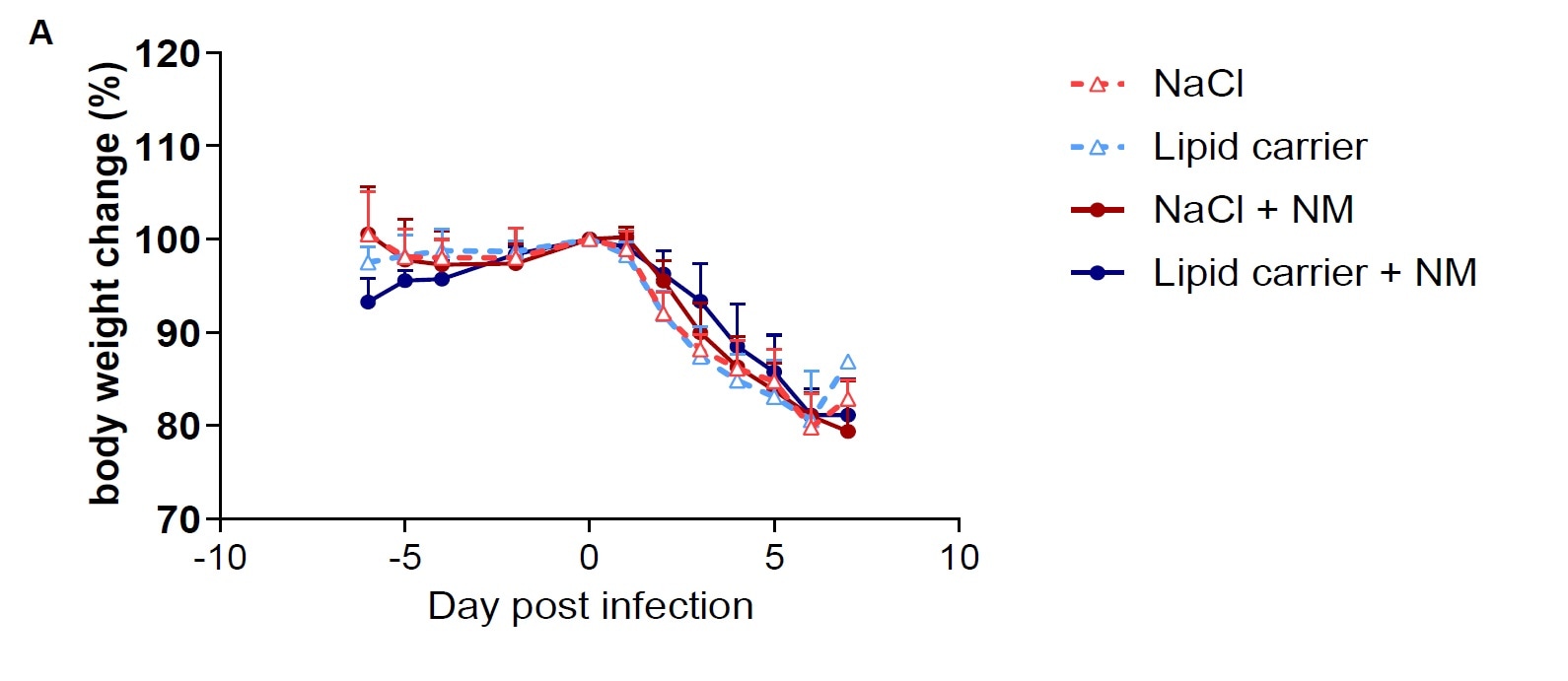
Bodyweight loss is not prevented by single Nafamostat mesylate +/- lipid carrier nasal application. Each 10 animals (8 animals from day 2 on) were pretreated by single nasal treatment application as indicated and daily weighed post nasal SARS-CoV2 virus inoculation. All animals were euthanized by day 7 p.i. or when they reached clinical endpoint (>20% weight loss relative to day 0). Error bars represent standard deviation. NM Nafamostat mesylate.
They found significantly reduced viral loads in the animals treated with NM in lipid when tested a day after infection. But, there was no difference in the viral loads after 3 days. All animals lost weight after infection, weight loss being an indication of disease severity.
Immunochemistry testing also showed that the nasal epithelium in all the animals, irrespective of the type of treatment they received, was strongly positive for the virus nucleoproteins. Examining the tissues of the nasal cavities showed inflammation and presence of mucus and in the nose. Furthermore, nasal epithelial tissues were found to be destroyed or severely damaged.
Viral load reduction temporary
Although there was a big decrease in the viral loads in the nose, this effect was temporary, and it did not affect the onset or severity of the disease. The addition of the lipid carrier also did not affect the viral loads much.
Although NM effectively prevented entry of SARS-CoV-2 in previous in vitro experiments, NM has an intravenous half-life of only eight minutes. Hence, a single application may not be enough to achieve sufficient protection. It is also likely that the hamster experiment did not simulate early infection, but rather the later stages of the infection – with the virus moving directly to the lungs from the nose and throat.
However, another experiment in ferrets with nasal application of lipopeptides showed positive results, where the dose and route of application were different. Thus, a different experimental design, along with the addition of lipopeptide with NM, may be an effective approach to reduce viral loads and disease severity.
Thus, the research group's experiments show that the application of NM in hamsters, with or without lipid carriers, can change nasal viral loads, which may reduce transmission risk and severity of the disease. In terms of its potential in COVID-19 therapy, NM is something that could perhaps be applied as an inhalator or a nasal spray. However, clinical success will depend on further testing and optimization of local stability.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.