The hunt for antivirals has reached an unprecedented pace in light of the coronavirus 2019 (COVID-19) pandemic. The causal agent for this disease – severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – has already infected over 52 million people worldwide and has subsequently claimed over 1.3 million lives. However, among the innumerable promising drug candidates that help battle the virus and mitigate its damage, none have yet made it to the clinic.
A team of researchers in the United States have explored an innovative direct-acting antiviral agent, peptide-conjugated morpholino oligomers (PPMO), and its efficacy against SARS-CoV-2. The researchers – from the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, and the Carlson College of Veterinary Medicine – have published their positive observations in the Journal of Antimicrobial Chemotherapy.
Peptide-conjugated morpholino oligomers (PPMO) are antisense compounds composed of a phosphorodiamidate morpholino oligomer covalently conjugated to a cell-penetrating peptide. They are water-soluble molecules that can seamlessly enter cells and bind to the target molecule. Because these are nuclease resistant and non-toxic at effective concentrations, PPMOs are good candidates for action against SARS-CoV-2. Previous studies have also shown that intranasally administered PPMO targeted against virus sequence has reduced viral titer and pathology in lung tissue.
PPMO are single-stranded nucleic acid analogs; the molecules can enter cells without assistance and interfere with gene expression through a steric blockade of targeted RNA.
In their study, the researchers used five PPMOs to target against various sites in the 5′UTR and polyprotein 1a/b translation start site (AUG) region of SARS-CoV-2. It is well known that the 5'UTR region of the coronavirus genome contains important sequences and structures that are essential in various aspects of the viral life cycle, including translation and RNA synthesis.
The researchers designed the PPMO to target the 5′UTR and the first translation start site region of SARS-CoV-2's positive-sense genomic RNA. They found that the PPMOs targeting the 5′ terminal region and the leader transcription regulatory sequence (TRS) region of genomic RNA were potent inhibitors of SARS-CoV-2 replication.
The study was conducted in Vero E-6 cells, a lineage of cells used in cell cultures predominantly in vaccines or research experiments. Each PPMO used in the study was thus assessed for its effect on the viability of uninfected cells and its inhibitory effect on the replication of SARS-CoV-2 in Vero cell cultures.
Two of the PPMOs (5′END-1 and 5′END-2) targeted the 5′ terminal region of the genome to interfere with the pre-initiation of the translation of genomic and subgenomic messenger RNAs (mRNAs). Two others targeted the TRS region; TRS is a 6–10 nucleotide sequence critical in the production of negative-strand mRNA templates during the mRNA synthesis. The fifth PPMO, named AUG, targets the AUG translation start site of SARS-CoV-2 1a/b polyprotein.
In this study, the negative control is a random sequence PPMO - to control for any non-specific binding of the PPMO. The designed negative control contains little significant homology to any primate, rodent or viral sequences.
They found that a dose-dependent antiviral assay and a quantitative cell viability assay produced not more than a 5% cytotoxic effect.
However, they observed that the inhibitory effect of the various PPMO on SARS-CoV-2 replication was highly effective, suppressing viral titers by 4–6 log10.
Out of the five antiviral PPMOs used in this study, four turned to show successful results. The researchers found in this study that the PPMOs targeting the 5′ terminal region or leader-TRS region were highly effective at inhibiting the growth of SARS-CoV-2. In contrast, the PPMO targeting the polyprotein 1a/b AUG translation start site region was less effective. The PPMOs also exhibited durable potency.
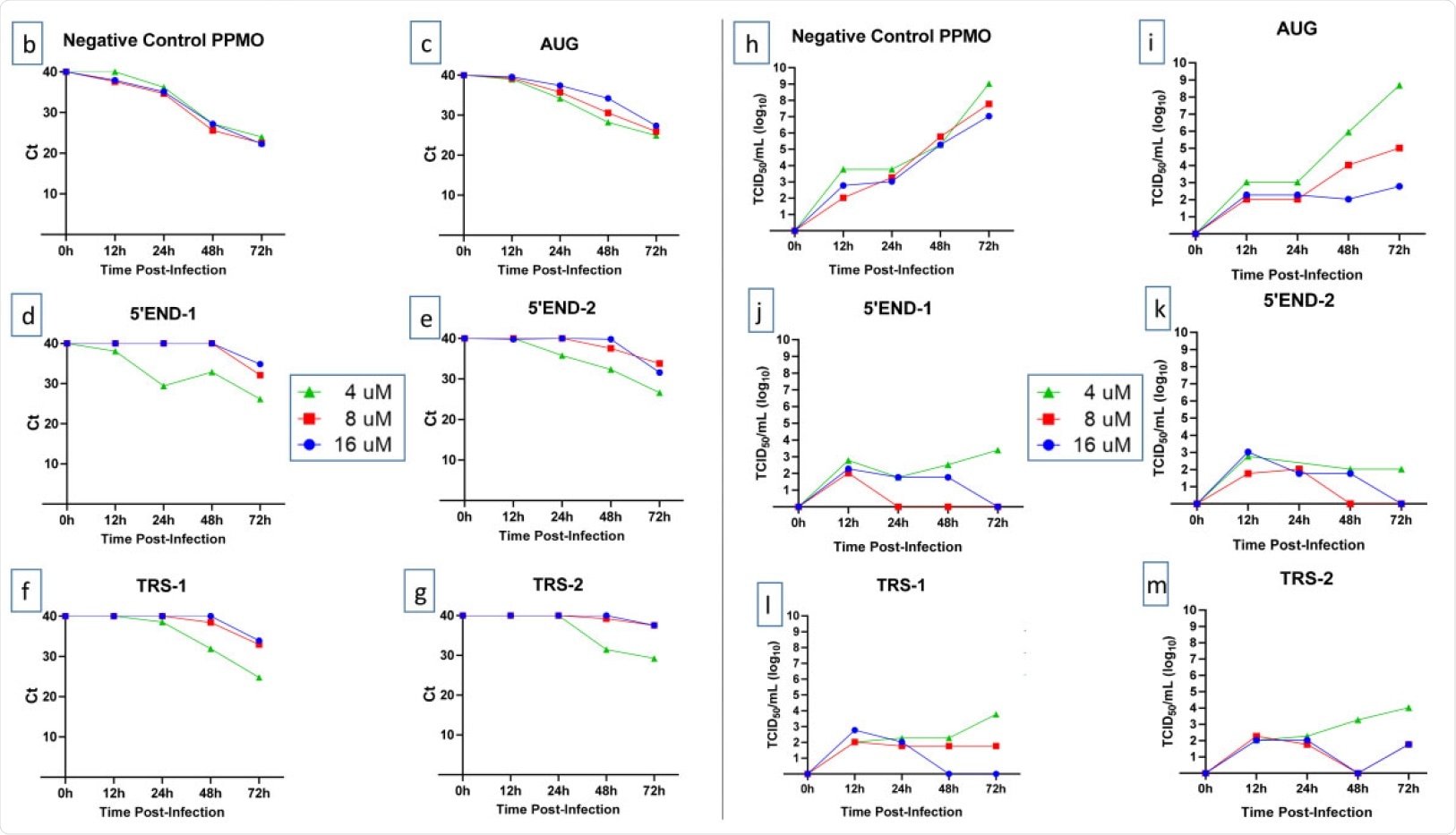
Effect of PPMO on cell viability and SARS-CoV-2 growth. (a) Evaluation of the effect of PPMO treatment on cellular ATP level, as an indicator of cell viability, was carried out using uninfected cells incubated for 48 h with increasing concentrations of the indicated PPMO. ATP levels were determined via luminescence readings and are shown compared with PBS-treated cells. For each PPMO concentration, triplicate samples were assayed and the mean±SD is shown. (b–m) Growth curves of SARS-CoV-2. Vero-E6 cells were treated with the indicated concentration of PPMO for 5 h before infection with SARS-CoV-2 (moi of 0.01), then incubated without PPMO after infection. Cell supernatants were collected at 12, 24, 48 and 72 h post-infection and analysed by qRT–PCR (b–g) or TCID50/mL endpoint dilution (h–m) using three technical repeats. Cells treated with PBS had titres similar to those shown for NC PPMO. The limit of virus detection for the TCID50 assay was 101/mL. This experiment was carried out twice, under similar conditions, yielding similar results, and the results from a single experiment are shown. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Overall, the study successfully demonstrated that the PPMOs targeted against SARS-CoV-2 readily enter cells and inhibit viral replication in a sequence-specific, dose-responsive, and non-toxic manner.
The findings here warrant further development of the 5′END and TRS PPMO to address SARS-CoV-2 infections. Maybe the hunt ends here with these antiviral molecules.