Researchers at the National Institutes of Health in Bethesda, Maryland, have shown how certain synthetic nanobodies bind to the agent that causes coronavirus disease 2019 (COVID-19) – a development that could help inform the design of therapies.
The team describes the X-ray crystal structures of three synthetic nanobodies (sybodies) that bind to the receptor-binding domain (RBD) of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Superposing the structures onto models of the viral spike protein revealed the different ways in which these sybodies attach to the RBD.
The RBD is the primary region on spike responsible for attaching to the host cell receptor angiotensin-converting enzyme 2 (ACE2).
David Margulies and colleagues say the structural studies suggest that a battery of sybodies or nanobodies have the potential to completely saturate the available RBD surface.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Examining the detailed structures of anti-viral antibodies
The SARS-CoV-2 infection process begins with the binding of the trimeric spike glycoprotein to ACE2. Examining the detailed structures of anti-viral antibodies can provide critical insights into how this viral adsorption and cell entry could be weakened to prevent or slow ongoing infection and communal spread.
Studies have recently indicated the value of camelid-derived single domain antibodies (nanobodies) or camelid-inspired synthetic libraries (sybodies) for generating multivalent constructs that could aid the development of effective treatments.
What did the current study involve?
The team reports four X-ray crystal structures of three sybodies (Sb16, Sb45 and Sb68) that bind to the receptor RBD of SARS-CoV-2. These sybodies have previously been shown to effectively inhibit the ACE2–RBD interaction and to neutralize viral infectivity.
To investigate the detailed topology of the interaction between these sybodies and the RBD, the researchers also determined the crystal structures of several complexes: the binary complexes of Sb16–RBD and Sb45–RBD, a ternary complex of Sb45–RBD–Sb68, and unliganded Sb16.
What did they find?
The Sb16–RBD complex shows that the antibody complementarity determining region 2 (CDR2) and CDR3 of Sb16 straddles the saddle-like region of the RBD surface that binds ACE2. The Sb45–RBD complex showed Sb45 straddles this region in the opposite orientation and frames the interface with CDR2 and CDR3. The CDR1 of both sybodies is located between the CDR2 and CDR3 loops. Superposition of the two structures showed that the CDR2 of Sb16 and CDR3 of Sb45 bind to the same epitope regions, while superposition of the ternary Sb45–RBD–Sb68 shows that Sb68 interacts at a completely different site on the RBD.
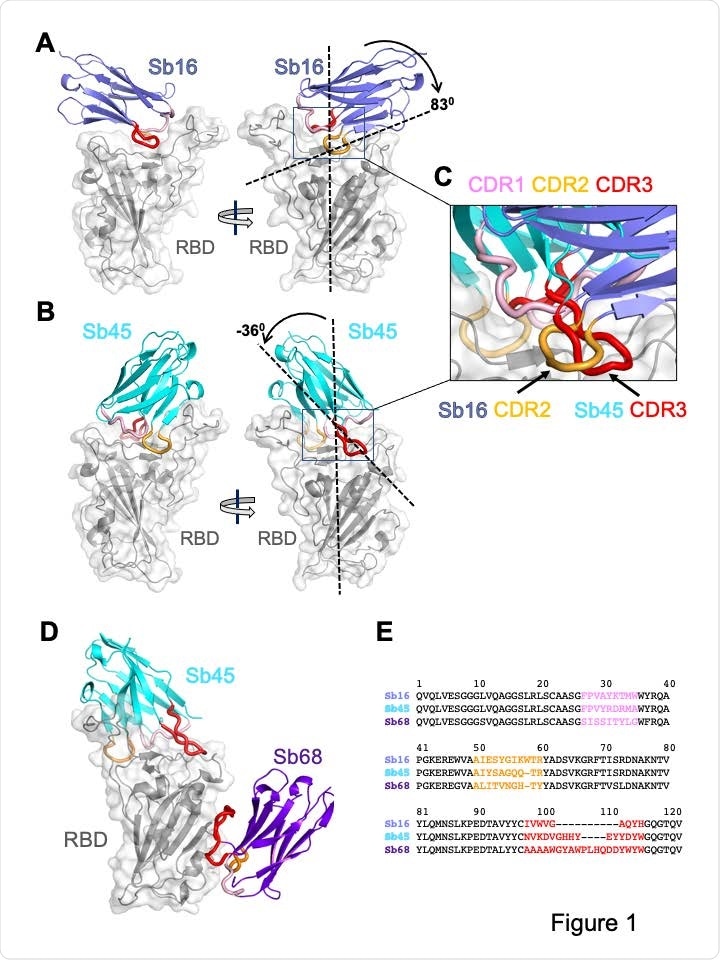
Overall structures of Sb16, Sb45 and Sb68 complexes with SARS-CoV-2 RBD. Ribbons (sybodies) and ribbons plus surface (RBD) representations of the complex of (A) Sb16 (slate) with RBD (grey) (7KGK); (B) Sb45 (cyan) with RBD (7KGJ), and (C) Sb45 and Sb68 (purple) with RBD (7KLW). Sb16-RBD and Sb45-RBD, superimposed based on the RBD are shown in (D) to highlight CDR loops, which are color coded as indicated. The CDR2 of Sb16 and CDR3 of Sb45 interact similarly with the RBD surface.
Margulies and colleagues say it is particularly interesting that while Sb45 CDR2 and CDR3 span the saddle-like region of the RBD, the distinct interactions of Sb68 and the RBD occur via the longer CDR3, with only minor involvement of CDR1 and CDR2.
To assess the structural basis underlying the ability of these three sybodies to block the RBD–ACE2 interaction, the team superposed each of the three sybody–RBD structures onto an ACE2–RBD structure. This revealed that Sb16 and Sb45 directly target the ACE2 binding site, potentially explaining their neutralizing capacity. On the other hand, Sb68 binds to the RBD at a site that seems to be non-competitive for ACE2 binding.
Sb16 and Sb45 cover more surface area than other known complexes
Analysis of the buried surface area (BSA) interfaces between RBD and ACE2 or the sybodies showed that Sb16 and Sb45 cover more surface area than ACE2 and other known sybody–RBD complexes. The smallest interface was observed for Sb68.
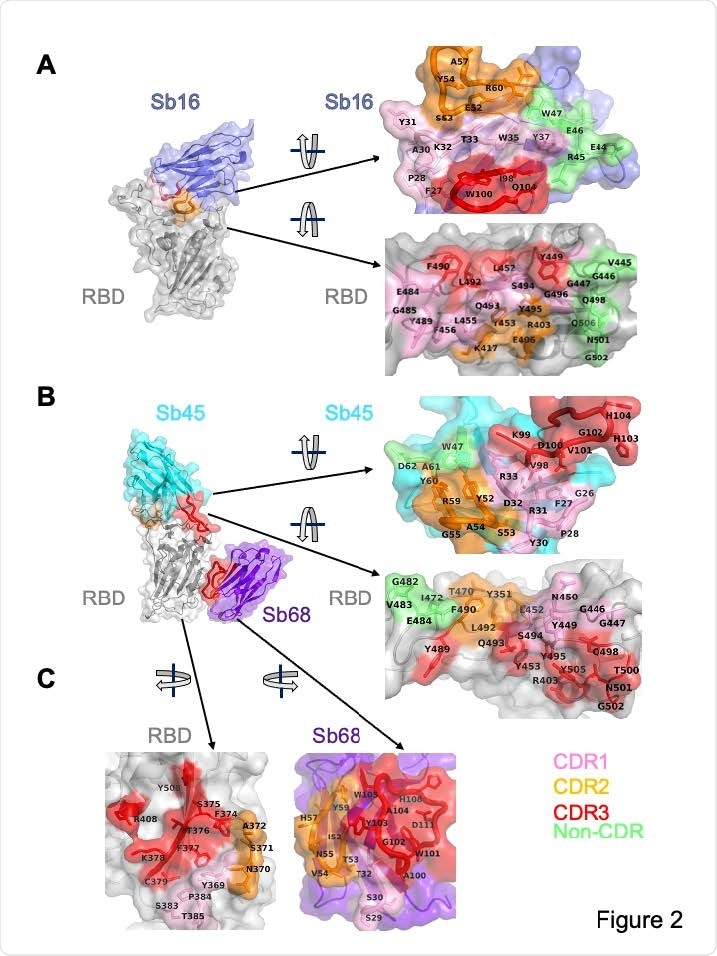
Interface and interaction of Sb16-RBD, (B) Sb45-RBD and (C) Sb68-RBD. CDR1, CDR2, CDR3 regions are painted pink, orange and red respectively. Additional non-CDR region contacting residues are colored lime. On the RBD surface, the epitopic residues that contact the sybodies are colored according to the sybody CDR.
Further analysis of the superposition of the Sb68–RBD complexes on ACE2–RBD showed that the Sb68 loop 40-44 clashes with amino acid side chains of ACE, while loop 61-64 clashes with the ACE2 carbohydrate N322, and loop 87-89 clashes with the ACE2 N546 carbohydrate.
Furthermore, molecular dynamics simulations of RBD–ACE2 indicated that the carbohydrates interacted directly with the RBD.
“Thus, the ability of Sb68 to impinge on ACE2 interaction with RBD likely involves the steric clash of the N322- and N546-linked glycans,” say the researchers.
The team says the findings demonstrate how the small size of sybodies or nanobodies can be advantageous in accessing epitopic regions of the spike.
“Overall, our structural studies not only define the Sb16, Sb45, and Sb68 epitopes at high resolution, they suggest that a battery of sybodies or nanobodies have the potential to saturate the available RBD surface,” writes Margulies and colleagues.
What are the implications of the study?
The researchers point out that the SARS-CoV-2 variant that recently emerged in the UK has multiple substitutions in the RBD and that, while this is expected to affect the footprint of Sb16 and Sb45, it would not have any effect on the Sb68 site.
“Thus, precise mapping of anti-RBD antibody, nanobody, and sybody epitopes, especially for those that are developed for clinical trials, has implications not only for mecha nistic understanding of the interactions of the RBD with ACE2, but also for evaluating the potential susceptibility of newly arising viral variants to currently administered vaccines and antibodies,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Margulies D, et al. Synthetic nanobody–SARS-CoV-2 receptor-binding domain structures identify distinct epitopes. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.01.27.428466, https://www.biorxiv.org/content/10.1101/2021.01.27.428466v1
- Peer reviewed and published scientific report.
Ahmad, Javeed, Jiansheng Jiang, Lisa F. Boyd, Allison Zeher, Rick Huang, Di Xia, Kannan Natarajan, and David H. Margulies. 2021. “Structures of Synthetic Nanobody–SARS-CoV-2 Receptor-Binding Domain Complexes Reveal Distinct Sites of Interaction.” Journal of Biological Chemistry 297 (4). https://doi.org/10.1016/j.jbc.2021.101202. https://www.jbc.org/article/S0021-9258(21)01004-8/fulltext.