The ability to rapidly detect diseases with high precision is of paramount importance, as evidenced by the current COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Researchers have developed a novel strategy for simplified single-particle digital assay based on DIgitAl plasMONic nanobubble Detection (DIAMOND) to meet this urgent and unmet requirement. This new technology provides rapid and ultrasensitive detection of viruses in a one-step homogeneous assay.
An interdisciplinary team from the University of Texas applied DIAMOND to the respiratory syncytial virus (RSV) diagnostics and demonstrated that DIAMOND is 150 times more sensitive than commercial lateral flow assays. They found that the measurements are completed within 2 minutes. The results of this study are published on the medRxiv* preprint server.
Digital assays involve single-molecule detection and absolute quantification. While the conventional assays, such as the enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction(PCR), are used extensively, digital assays are advancing and advantageous.
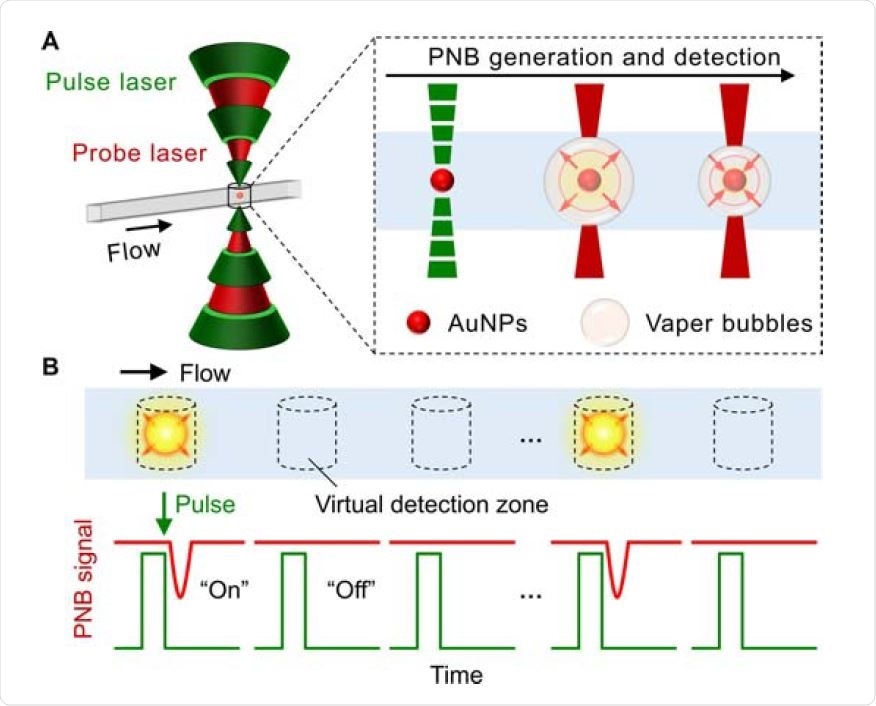
The schematics illustration for the concept of DIAMOND. (A) The spectroscopy-based signal generation and detection. The gold nanoparticles (AuNPs) as labels are used for the generation of the plasmonic nanobubbles (PNBs) by short laser pulses and subsequently detected by a secondary probe laser due to the optical scattering. (B) The detection principle based on optofluidic scanning of a sample flowing through. The “on” and “off” refers to the positive and negative PNB signals representing for the presence or absence of targets.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
In a digital assay, the analyte is partitioned into microwells or emulsion droplets as small compartments for independent signal amplification and digital counting. The sensitivity enhancement is enhanced up to 103-fold over the conventional assays.
However, digital assays have suffered from complex assay operations. Digital sensing platforms that are based on micro/nanoparticles overcome these limiting challenges. Single-particle detection examines the changes in a single particle upon recognizing the target molecules. These assays include bright/dark-field imaging, interferometric or fluorescent imaging, surface-enhanced Raman scattering, surface plasmon resonance microscopy imaging, and particle mobility tracking. However, these techniques include cumbersome particle purification and advanced imaging protocols that may limit their widespread use.
The DIAMOND uses gold nanoparticles (AuNP) as labels and an optofluidic set-up, it achieves compartment-free digital counting and works on homogeneous assays without separation and amplification steps.
.jpg)
Detection of SiO2 beads in a homogeneous assay by DIAMOND. (A) Schematic of a homogeneous assay of SiO2 beads by AuNPs as a pair of targets and probes at room temperature (RT). Lower panel shows that when bead concentrations are insufficient to induce the color change, DIAMOND can detect the PNB signals. (B) TEM image of SiO2-AuNPs conjugates. Scale bar in inset is 200 nm. (C) Representative PNB signal traces (100 pulses) for the assay solutions. Schematics represent the assay information that show the different λ of SiO2 beads and the same λ of AuNPs. (D) Bivariate plot of amplitude and AUC extracted from 3,000 pulses for the assay solutions with different λ of SiO2 beads. Dashed lines indicate the positions of thresholds calculated from the control sample. (E) Quantification of SiO2 bead concentration as a function of frequency counting (fon). Error bars indicate the standard deviations of three independent measurements, and the LOD was calculated as 3 standard deviations of the control dividing the slope of regression line. (F) Correlation between the background-subtracted frequency (fon’) and Poisson probability (P).
Plasmonic nanobubbles (PNBs) refer to the vapor bubbles generated by short laser pulse excitation of plasmonic nanoparticles (NPs) and amplify their intrinsic scattering for the detection by a secondary probe laser. The nanobubbles last for nanoseconds and are sensitive to nanoparticles' physical parameters such as sizes, shape, concentration, and clustering state.
The researchers designed an optofluidic set-up to flow the nanoparticles suspension in a micro-capillary using these unique properties. A focused laser beam probes a microscale "virtual compartment" of about 16 pL and detects the single particles' PNB generation.
There is no cross-talk between laser pulses because the PNBs are transient.
This allows for "on" and "off" signal counting in a compartment-free manner. The researchers demonstrated robust single nanoparticle counting and sensitive detection of large particles in a strong background of small particles (1 in 240).
The researchers discussed the dependence of the PNB generation on the laser fluence. They performed the DIAMOND test in two modes by modulating the laser fluence above and below the PNB generation threshold, referred to as above- and below-threshold modes.
In the set-up, they implemented DIAMOND in a homogeneous assay that uses plasmonic gold NPs (AuNPs) as labels - without additional separation or amplification steps. The researchers have presented a comparison of DIAMOND with other assays.
Using silica (SiO2) beads as targets, they demonstrated that DIAMOND has a sub-femtomolar detection limit, providing absolute quantification.
The researchers demonstrated the efficacy of DIAMOND with a sensitivity enhancement of ~333-fold over the colorimetric result and of ~150-fold over the state-of-the-art lateral flow assay (LFA) to detect the respiratory syncytial virus (RSV) with the sample-to-answer time <2 minutes. The RSV is the major respiratory pathogen.
The researchers also applied DIAMOND to quantify the RSV in the transporting medium used to collect nasal swab samples, demonstrating the high sensitivity and thus the potential clinical use.
The researchers have developed DIAMOND as a novel method for single-particle digital counting in an AuNP homogeneous assay. They also demonstrated that DIAMOND allows single nanoparticle detection and identification of heterogeneity from a uniform population.
This study demonstrated the single-particle digital plasmonic nanobubble detection, allowing rapid and ultrasensitive detection of viruses in a one-step homogeneous assay. It is a method with new possibilities that would facilitate rapid and ultrasensitive diagnostics.
Therefore, DIAMOND opens new possibilities to develop separation-, amplification-, and compartment-free single-particle digital assays and facilitate rapid and ultrasensitive diagnostic platforms, the researchers write.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Single-Particle Counting Based on Digital Plasmonic Nanobubble Detection for Rapid and Ultrasensitive Diagnostics, Yaning Liu, Haihang Ye, HoangDinh Huynh, Peiyuan Kang, Chen Xie, Jeffrey S Kahn, Zhenpeng Qin, medRxiv 2021.02.18.21252027; doi: https://doi.org/10.1101/2021.02.18.21252027, https://www.medrxiv.org/content/10.1101/2021.02.18.21252027v1
- Peer reviewed and published scientific report.
Liu, Yaning, Haihang Ye, HoangDinh Huynh, Chen Xie, Peiyuan Kang, Jeffrey S. Kahn, and Zhenpeng Qin. 2022. “Digital Plasmonic Nanobubble Detection for Rapid and Ultrasensitive Virus Diagnostics.” Nature Communications 13 (1). https://doi.org/10.1038/s41467-022-29025-w. https://www.nature.com/articles/s41467-022-29025-w.