With the current coronavirus disease 2019 (COVID-19) pandemic spreading to almost every country in the world, lockdowns, social distancing and other non-pharmaceutical interventions (NPIs) have been almost the only effective measures against the spread of the causative pathogen, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
This has stimulated the accelerated development of vaccines as the definitive way out of this global crisis.
While several vaccines have gained emergency use authorization by multiple regulatory authorities in Europe, Asia and the UK, the continuing and rapid emergence of new variants with mutants that confer partial vaccine resistance pose a severe challenge to the goal of achieving population-level immunity.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
A new study by researchers at Mahidol University in Bangkok, Thailand, reports a COVID-19 vaccine candidate called HexaPro in producing neutralizing antibodies against the SARS-CoV-2 spike protein in an animal model. The team released its findings on the bioRxiv* preprint server.
Spike-mediated entry
The novel coronavirus mediates entry into the host cell through its spike protein. This bears a receptor-binding domain (RBD) that binds to the angiotensin-converting enzyme 2 (ACE2) receptor on the host cell. This binding triggers a conformational change in the spike protein, which allows viral infiltration of the cell, so that active replication can begin.
The spike protein is also the dominant immunogen of this virus and has been the chief focus of most vaccine development efforts. To facilitate this, the prefusion conformation of the spike has been stabilized by the introduction of two successive prolines (S-2P), at the loop intervening between the central helix and the heptad repeat 1 of this protein.
S-2P variants with a C-terminal trimerization domain have increased immunogenicity, and these have become the norm in the currently used vaccines from Moderna, Pfizer and Astra-Zeneca.
Spike subunit
The current paper discusses a COVID-19 vaccine candidate developed based on a recently reported proline-stabilized prefusion spike ectodomain subunit – HexaPro.
The researchers found that it induces a robust neutralizing antibody response in mice against SARS-CoV-2, and could be further developed as a second-generation coronavirus vaccine.
The subunit vaccine is based on the spike ectodomain that has six substitutions with proline as well as a GSAS substitution at the furin cleavage site, besides the C-terminal fold on the trimerization domain.
Induction of neutralizing activity
The mice were inoculated intramuscularly with a prime-boost protocol. A microneutralization assay showed that serum from immunized mice at two weeks after the booster dose induced high titers of neutralizing antibodies. This level was sustained for eight weeks at least.
The subunit spike vaccine candidate HexaPro produced powerful neutralizing responses. This spike protein ectodomain has four additional proline substitutions to stabilize the prefusion conformation still further. This is within the S2 domain of the stabilized S-2P spike.
In this novel variant of the prefusion spike, a third of the trimeric spike RBDs are in the ‘up’ or open conformation. As a result, two RBDs are exposed instead of the single exposed RBD in the S-2P spike. The increased stability stimulated the current exploration of HexaPro as a potential vaccine candidate against COVID-19.
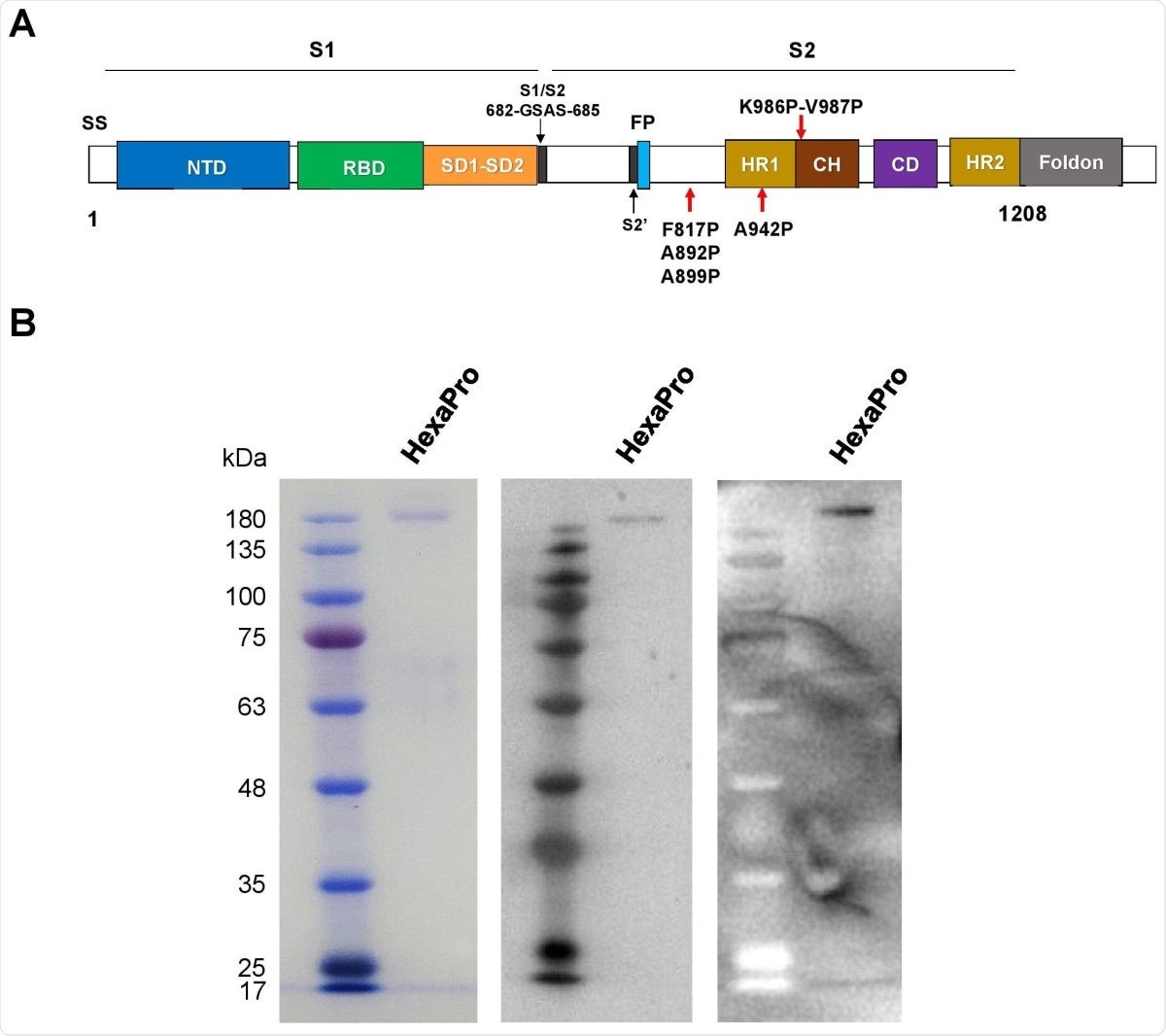
The recombinant SARS-CoV-2 HexaPro spike protein. (A) Schematic representation of the prefusionstabilized SARS-CoV-2 HexaPro ectodomain showing the S1 and S2 subunits. Four additional proline substitutions from S-2P construct are indicated by the red arrows shown below the construct. (B) The HexaPro protein expressed in HEK293T cells was purified and characterized by SDS-PAGE (left), western blot using a commercial anti-RBD (middle), and western blot using pooled convalescence sera (right).
The use of alum (aluminum hydroxide) in vaccines is associated with increased dendritic cell and T cell activity, typical of inflammation. A recent phase 1 trial used alum with the inactivated SARS-CoV-2 virus BBV152, and other clinical trials using subunit SARS-CoV-2 vaccines or inactivated SARS-CoV-2 vaccines with alum are also currently ongoing.
Besides the use of alum as an adjuvant, the current paper describes the use of a low priming dose and a high booster dose. This is supported by numerous studies in which this profile is known to induce a stronger immune response. One prominent study of interest is the randomized controlled trial of the approved Oxford-Astra-Zeneca vaccine.
Higher doses of an immunogenic antigen at the time of the priming dose may suppress effector cell recruitment. Conversely, immune memory cells are found to be induced by lower prime doses. Thus, a low prime-high booster dose regimen would seem to be more suitable for the induction of a durable anamnestic immune response.
What are the implications?
The current study shows that the HexaPro stabilized prefusion spike ectodomain is a highly stable conformation. Its production is suited to rapid and cost-effective production. It is also conveniently transported, overcoming logistical barriers.
In view of the strongly neutralizing response elicited in immunized animals by this subunit vaccine candidate, the researchers suggest that this should be taken further. For instance, selected mutations should be introduced to induce effective immunization against different SARS-CoV-2 variants. Other vaccine platforms, such as mRNA, viral vector and DNA vaccines, may also use this antigen effectively.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Seephetdee, C. et al. (2021). Mice immunized with the vaccine candidate HexaPro spike produce neutralizing antibodies against SARS-CoV-2. bioRxiv preprint. doi: https://doi.org/10.1101/2021.02.27.433054, https://www.biorxiv.org/content/10.1101/2021.02.27.433054v1
- Peer reviewed and published scientific report.
Seephetdee, Chotiwat, Nattawut Buasri, Kanit Bhukhai, Kitima Srisanga, Suwimon Manopwisedjaroen, Sarat Lertjintanakit, Nut Phueakphud, et al. 2021. “Mice Immunized with the Vaccine Candidate HexaPro Spike Produce Neutralizing Antibodies against SARS-CoV-2.” Vaccines 9 (5): 498. https://doi.org/10.3390/vaccines9050498. https://www.mdpi.com/2076-393X/9/5/498.