Researchers in Israel have conducted a study showing that the immunity provided against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes coronavirus disease (COVID-19) – is similar following natural infection or immunization with the Pfizer-BioNTech BNT162b2 vaccine.
The researchers from the Israel Institute of Technology, The Hebrew University of Jerusalem, and Sheba Medical Center say the findings raise the question of whether it is necessary to vaccinate individuals who have previously been infected with SARS-CoV-2.
The team’s analysis of individual-level data for the entire population of Israel found that both prior infection and vaccination were highly effective at preventing subsequent SARS-CoV-2 infection, hospitalization due to COVID-19, and severe disease.
“This is the first large-scale study to explore the protection due to prior SARS-CoV-2 infection compared with the Pfizer BNT162b2 vaccine,” says Yair Goldberg and colleagues.
“Our results question the need to vaccinate previously-infected individuals,” they write.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
More about the vaccination program in Israel
Israel is in the later stages of rolling out vaccination to protect against SARS-CoV-2 infection and reduce the number of COVID-19 cases.
The vaccination program was first launched in Israel on December 20th, 2020.
The Pfizer-BioNTech BNT162b2 vaccine was initially made freely available to those aged more than 60 years, followed by nursing home residents, healthcare workers, patients with severe comorbidities, and eventually younger age groups.
From February 6th, 2021, the vaccine was also made available to all individuals aged 16 or older with no history of previous SARS-CoV-2 infection.
By March 20th, 2021, 77% of the eligible population in Israel had been vaccinated.
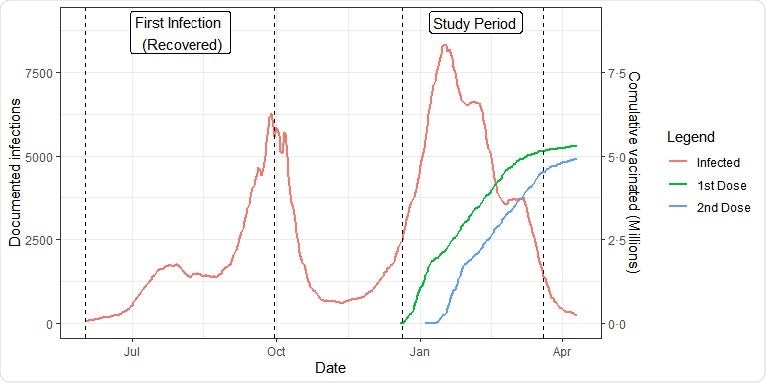
Population dynamics. Documented new infections and cumulative vaccinated persons by date. The study period and the infection period of the recovered cohorts are marked by vertical lines.
Previous studies showed the efficacy of both prior infection and vaccination
Recent studies have shown that vaccination substantially reduces SARS-CoV-2 viral load and the number of severe COVID-19 cases.
“These encouraging initial results are based on a short follow-up of vaccinated individuals,” says Goldberg and colleagues.
However, studies have also shown that SARS-CoV-2 infection provides protection against reinfection with the virus.
The researchers say that the worldwide shortage of vaccines, while the pandemic is still uncontrolled, has meant that many governments now face the dilemma of whether to vaccinate people who have already been infected.
“Understanding the level of protection of previous infection compared to that of vaccination is critical for policymaking,” they write.
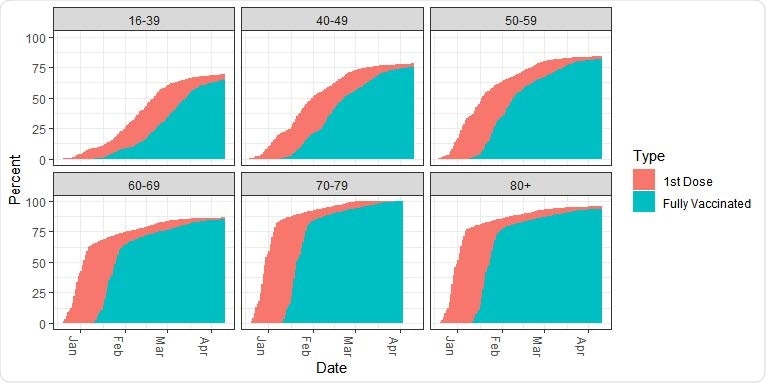
Vaccination by age. Percent of individuals vaccinated with the first and the second dose, by age group. The vaccination initiated in the 60+ age group.
What did the researchers do?
The researchers analyzed individual-level data collected by the Israel Ministry of Health and the Israel Central Bureau of Statistics on people aged 16 and older across the entire population of Israel.
The database included demographic data and information on dates of first and second vaccination, as well as the dates and results of all polymerase chain reaction (PCR) tests for SARS-CoV-2 that were performed between March 1st, 2020 and March 20th, 2021.
For individuals with a positive PCR test, data were available on symptoms, disease course (hospitalization, severe disease, and death), and the maximum disease severity status.
To estimate the efficacy of the BNT162b2 vaccine or prior infection in reducing documented SARS-CoV-2 infection and severe COVID-19, the team focused on four cohorts:
- Unvaccinated and previously uninfected individuals
- Vaccinated individuals who were followed from the day of the first dose to day six after the second dose
- Vaccinated individuals who were followed from one week after the second dose onwards
- Unvaccinated recovered individuals who had previously been infected with SARS-CoV-2 between June 1st and September 30th, 2020.
All efficacies of vaccination or previous infection were compared with the unvaccinated, previously uninfected cohort for the following outcomes: documented infection, hospitalization, severe disease, and death.
Follow-up was carried out for an extended duration
The data are based on follow-up of the four cohorts from December 20th, 2020, until March 20th, 2021, with more than 573 million person-days of follow-up.
“The study included follow-up of the population for a period of three months, allowing follow-up of the fully vaccinated cohort over an extended duration,” write the researchers.
During this time, 306,712 individuals tested PCR-positive for infection (5·4 infections per 10,000 person-days). Of these individuals, 14,019 (4·6%) required hospitalization, 8,463 (2·8%) developed severe disease, and 2,727 (0·9%) died.
What were the efficacy results?
The overall estimated efficacy of vaccination was 92·8% for documented infection, 94.2% for hospitalization, 94.4% for severe illness and 93.7% for death.
Similarly, the overall estimated level of protection among individuals with prior SARS-CoV-2 infection was 94.% for documented infection, 94.1% for hospitalization and 96·4% for severe illness.
Since only one death occurred in the recovered cohort, protection against death following prior infection was not estimated.
Findings raise the question of whether to vaccinate recently infected individuals
The researchers say the study suggests that both the BNT162b2 vaccine and prior SARS-CoV-2 infection are effective against both subsequent SARS-CoV-2 infection and other COVID-19–related outcomes.
“Moreover, the effectiveness seems similar for both cohorts,” they add.
This raises the question of whether it is necessary to vaccinate individuals who have recently been infected with SARS-CoV-2, concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Goldberg Y, et al. Protection of previous SARS-CoV-2 infection is similar to that of BNT162b2 vaccine protection: A three-month nationwide experience from Israel. medRxiv, 2021. doi: https://doi.org/10.1101/2021.04.20.21255670, https://www.medrxiv.org/content/10.1101/2021.04.20.21255670v1
- Peer reviewed and published scientific report.
Goldberg, Yair, Micha Mandel, Yonatan Woodbridge, Ronen Fluss, Ilya Novikov, Rami Yaari, Arnona Ziv, Laurence Freedman, and Amit Huppert. 2022. “Similarity of Protection Conferred by Previous SARS-CoV-2 Infection and by BNT162b2 Vaccine: A 3-Month Nationwide Experience from Israel.” American Journal of Epidemiology 191 (8): 1420–28. https://doi.org/10.1093/aje/kwac060. https://academic.oup.com/aje/article/191/8/1420/6556183.