The coronavirus disease (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continues to spread across the globe. The virus has infected over 165 million people worldwide, and of those, over 3.43 million have died. SARS-CoV-2 is a virus of the species severe acute respiratory syndrome-related coronavirus (SARSr-CoV). It appears to have zoonotic origins and exhibits close genetic affinity to bat coronaviruses.
As the pandemic continues, the SARS-CoV-2 has evolved to acquire mutations on the spike protein, the part of the virus that latches onto cells to infect them, to enhance the virus’s ability to bind to human cells or evade antibodies. As a public health measure, efficient screening methods are necessary to determine the functional effects of new sequence variants.
Researchers at New York University showed that the structural modeling of SARS-CoV-2 spike protein binding to the human angiotensin-converting enzyme 2 (ACE2) receptor, the initial step in host-cell entry, predicts many novel variant combinations with enhanced binding affinities. This means that the new computational method can identify high-affinity mutations and effectively assess mutations in the spike protein and ACE2.
The study, published in the Journal of Molecular Biology, shows promise in detecting potential new variants that can cause the faster spread of COVID-19. The recent mutations and variants detected have caused the rapid spread of the infection in many countries. Finding the potential mutation combinations can be a tool for predicting future ones and, at the same time, for preventing them early.
SARS-CoV-2 mutation
In the SARS-CoV-2, the spike protein acts as the key for the virus to bind to and enter human cells. Several structural proteins are embedded in the viral envelope. The S protein contains the S1 and S2 subunits. The S1 unit has the receptor-binding domain (RBD), while the S2 anchors the spike protein to the viral envelope.
Most coronaviruses, including the SARS-CoV-2, use the RBD to enter host cells by binding to the human angiotensin-converting enzyme 2 (ACE2) receptors, stimulating virus-host cell fusion. It is also in the spike protein that mutations occur.
Understanding viral mutations will influence how scientists assess virus-host interactions and develop intervention strategies.
In viral outbreaks, mutations occur for the virus to evade the immune system. Recent modifications during the COVID-19 pandemic have caused a widespread surge in cases. The United Kingdom, Brazil, South Africa, and India all experienced skyrocketing cases due to emerging SARS-CoV-2 variants, making it hard to attain vaccination success.
Analysis of SARS-CoV-2 genome sequences suggests thousands of mutations, but their health implications remain unclear. It is essential to develop methods that can efficiently detect COVID-19 mutations that can serve as surveillance tools for new emerging strains. This can also help in developing novel vaccines.
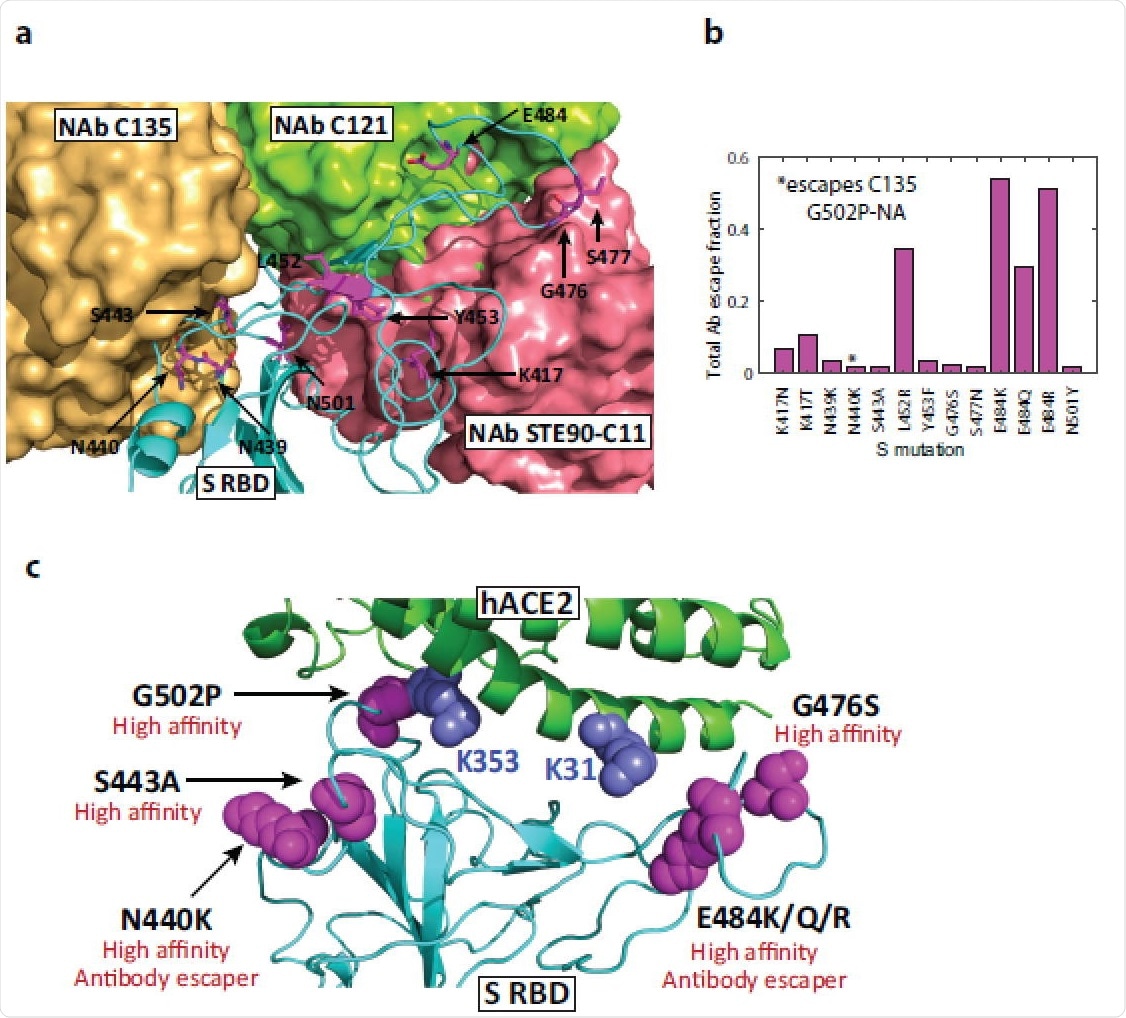
High-affinity mutations and antibody binding sites in Spike RBD. (a) Structure of WT S RBD superimposed with three neutralizing antibody structures (C121, 7K8X; C135, 7K8Z; STE90-C11, 7B3O). Residues predicted to harbor high-affinity variants and fast-spreading mutations are highlighted, illustrating that mutations in antibody binding sites have the potential to reduce their efficacy. Bound C135 and STE90-C11 antibody structures partially overlap. (b) Antibody escape of mutations as measured using the total escape fraction, i.e., the sum of all escape fractions across 10 antibodies tested using deep mutagenesis. (c) Summary of significant high-affinity mutations in S and hACE2 proteins and their functional implications.
Screening mutations
The researchers applied a structural modeling approach for more efficient screening of numerous mutant SARS-CoV-2 S-ACE2 complexing to arrive at the study findings. They computed their binding affinities that have been shown as a reliable measure of the viral potential to infect host cells. The novel approach aims to fill the gap between sequence-based analysis and laboratory confirmation of mutations.
The study also showed that the structural modeling of the S protein binding to the ACE2 receptor helps predict many novel variant combinations with enhanced binding affinities.
By focusing on natural variants at the Spike-ACE2 interface and studying more than 700 mutant complexes, the researchers revealed that high-affinity Spike mutations tend to cluster near known human ACE2 recognition sites. Applying structural analysis to highly transmissible variants, the team found that circulating point mutations, such as N501Y, E484K, and S477N, form high-affinity complexes.
The team also noted that when predicted affinities and available antibody escape data are merged, fast-spreading variants exploit combinatorial mutations with improved affinity and antibody resistance. They looked at single, double, and triple mutations in the critical spike interface region. The model analysis shows the spike variants S477N, N501Y, and S477N + E484K and E484K + N501Y, the fast-spreading double mutants found in the U.S., the U.K., Brazil, and South Africa have enhanced binding to the ACE2 receptor.
The results showed that the E484K and E484Q mutations found in fast-spreading variants could bind more strongly to the ACE2 receptor.
“Interestingly, our models predict that the triple mutation K417T/E484K/N501Y, which arose in the ancestral lineage E484K/N501Y, has about the same affinity as WT (wild type), although its frequency is increasing around the globe,” the researchers discussed.
Based on the study findings, the team proposed that structural modeling of novel variants as they emerge could help predict which mutations may pose the greatest threat amid the current global health crisis.