The development of specific antibodies in the blood serum against a particular pathogen as a result of immunization or infection is known as seroconversion.
Research laboratories buy seroconversion panels to perform testing on samples collected from a range of individuals, and in COVID-19, they are used to investigate antibody responses, providing valuable data regarding a vaccine's effectiveness.
In a paper recently uploaded to the preprint server medRxiv* by Belda et al. (June 27th, 2021) two seroconversion panels containing the sera of individuals that received the Moderna (mRNA-1273) vaccine are compared, and the impact on neutralizing IgG antibody titers in these individuals is analyzed.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
How was the study performed?
Two seroconversion panels (termed H and G) were utilized in the study to characterize the dynamics of SARS-CoV-2 Immunoglobulin G (IgG) following administration of the Moderna vaccine, both of which were sourced from Access Biologicals, USA.
The samples totaled 45 participants and were collected two days before the first dose of the vaccine, two days prior to the second dose, and two weeks after the final dose.
The samples were analyzed by enzyme-linked immunosorbent assay (ELISA) and chemiluminescent immunoassay (CLIA) , which are concerned with detecting IgG against the S1 and S2 spike protein or S1 spike protein only, respectively.
How did the vaccine influence IgG titers?
Pre-vaccination, two of fifteen individuals in seroconversion panel G had detectable levels of IgG by both chemiluminescence immunoassay (CLIA) and enzyme-linked immunosorbent assay (ELISA), suggesting that they had previously been infected with SARS-CoV-2. Seroconversion panel H contained 30 individuals, and pre-vaccination six of these had detectable levels of IgG, assessed by CLIA.
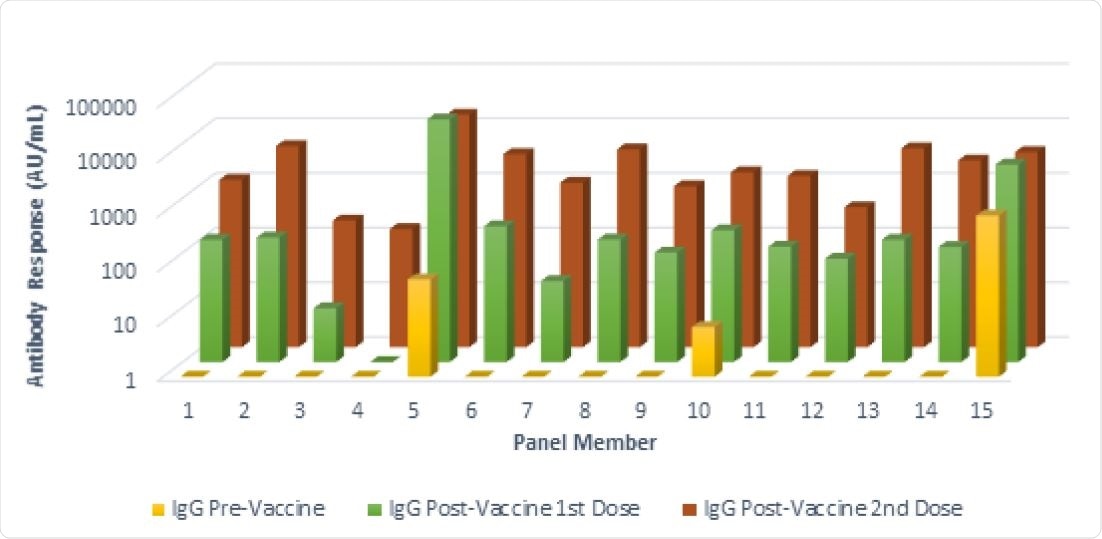
Antibody responses in a seroconversion panel (Panel G) of 15 subjects before and after vaccination with two doses of the mRNA-1273 SARS-CoV-2 vaccine (Moderna, Cambridge, MA, USA). These results were obtained using a chemiluminescent immunoassay. (AU/mL) Arbitrary Unit. Responses ≥ 15.0 units were considered positive. The front row shows results from samples collected prior to the first vaccination. The middle row shows the results from samples collected after the first vaccination and prior to the second vaccination. The back row shows the results from samples collected after the second vaccination.
After receiving the first dose of the vaccine, panel G showed 13 (of 15) positive results, while panel H showed 25 (of 30). A negative result is defined as being below the IgG titer cut-off threshold set by the authors.
Of the negative results following the administration of the first dose of the vaccine four of seven were aged over 70, likely due to a weakened immune response in these individuals.
After receiving the second dose of the vaccine, all 45 individuals represented by the seroconversion panels in the study had positive results, and there was a good quantitative agreement between CLIA and ELISA.
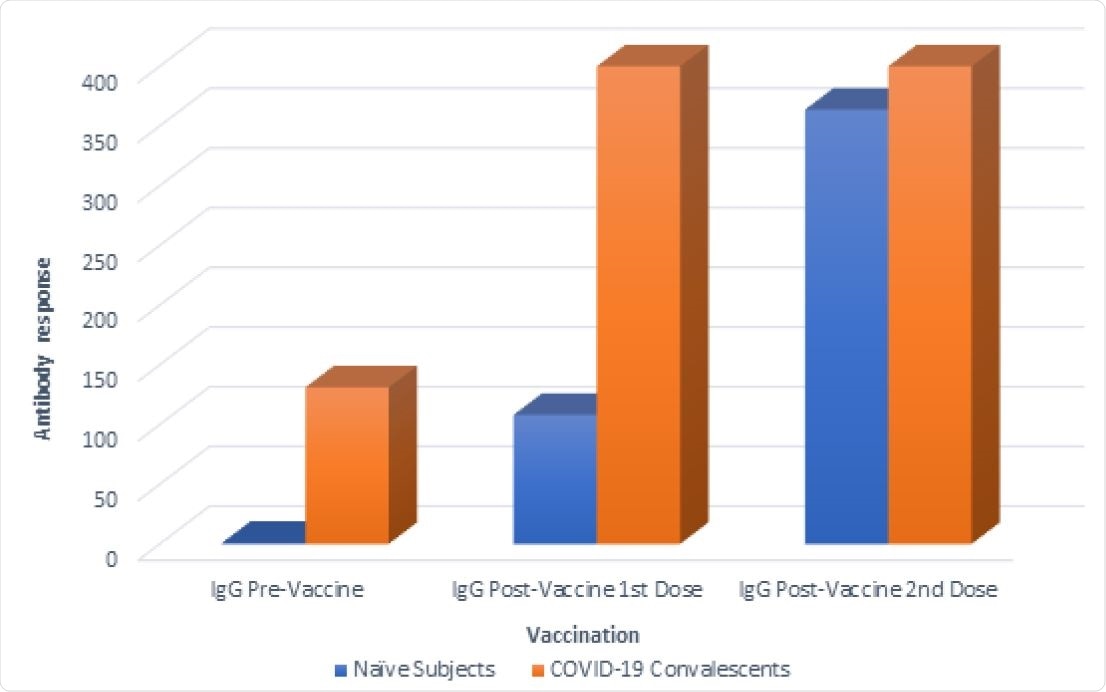
Comparison of antibody responses between naïve subject versus COVID-19 convalescent subjects prior to and after vaccination. Antibody responses were measured in samples collected prior to the first vaccination, after the first vaccination and prior to the second vaccination and after the second vaccination with the mRNA-1273 SARS-CoV-2 vaccine (Moderna, Cambridge, MA, USA). These results were obtained using a chemiluminescent immunoassay. (AU/mL) Arbitrary Unit. Responses ≥ 15.0 (AU/mL) were considered positive; values greater than the assay range (up to 400 AU/ml) were recorded as 400 AU/ml.
The group compared the difference in antibody response to the vaccine between COVID-19 naïve individuals and those that had previously been infected with SARS-CoV-2, as identified by having had high IgG levels pre-vaccination.
In the latter group, IgG levels rose from 131.2 (arbitrary units per mL) before the vaccine to 400 after the first dose, compared with a value of 108 achieved in COVID-19 naïve individuals after the first dose.
Following administration of the second dose, both naïve and COVID-19 experienced groups had similar IgG levels of around 400 units per mL, suggesting that two vaccine doses are approximately equivalent in IgG response to prior SARS-CoV-2 exposure and a single dose. This has been supported by a number of other studies and has been linked to a higher level of SARS-CoV-2 specific memory B cells in these individuals, consistent with prior infection.
The authors support a serostatus prioritization methodology in the context of a global vaccine shortage given these observations, in that those with convalescent plasma could instead receive just a single dose of the vaccine to equal effect as two.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Seroconversion panels demonstrate anti-SARS-CoV-2 antibody development after administration of the mRNA-1273 vaccine Francisco Belda, Oscar Mora, Monica Lopez-Martinez, Nerea Torres, Ana Vivanco, Rebecca Christie, Michael Crowley medRxiv 2021.06.20.21258152; doi: https://doi.org/10.1101/2021.06.20.21258152, https://www.medrxiv.org/content/10.1101/2021.06.20.21258152v1
- Peer reviewed and published scientific report.
Belda, Francisco, Oscar Mora, Monica Lopez Martinez, Nerea Torres, Ana Vivanco, Rebecca Christie, and Michael Crowley. 2022. “Seroconversion Panels Demonstrate Anti-SARS-CoV-2 Antibody Development after Administration of the MRNA-1273 Vaccine.” Vaccine, April. https://doi.org/10.1016/j.vaccine.2022.04.006. https://www.sciencedirect.com/science/article/pii/S0264410X22004194.