Immune correlates of protection can be utilized as a potent tool to fight against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. Levels of immune response, such as numbers of antibodies, can be used to predict the effectiveness and efficacy of vaccines through immune correlates.
In addition, correlates data can be obtained from automated in-vitro studies or blood samples much quicker than data gained through clinical trials or observational studies, which allows for the communication of vital information such as optimal vaccine dose and how new variants react to vaccines to be at a much more rapid pace.
Previous research has utilized ground-breaking frameworks to predict the efficacy of COVID-19 vaccines using live-virus titers, quantified in plasma collected from vaccinated volunteers. This research used a combination of data collected from different trials, which gives strength to the framework.
However, there may be issues in applying these frameworks in certain circumstances because another important factor to consider with vaccination is real-life effectiveness, and these frameworks are focused on the efficacy of vaccines from clinical trials.
Moreover, given that there is a 5-to-12-week interval between the first and second dose, it is vital to know the levels of protection acquired from a single dose, whereas these frameworks apply the assumption of full vaccination.
In order to address these issues, scientists from Xitific LTD and IDEAPharma in the UK proposed a novel approach to the framework of immune correlates, built upon the previous research. The framework formulated by the authors was developed for the mRNA-based vaccinations but could be applied to the other variations.
This study used estimates from the Pfizer-BioNTech phase 3 trial and seven large observational studies to model symptomatic vaccine effectiveness.
A preprint version of this study, which is yet to undergo peer review, is available on the medRxiv* server.
Does age-related immune response impact vaccine effectiveness?
A person’s age can impact their immune response, leading to substantial differences in vaccine effectiveness.
The authors compared neutralization data per dose cohort in individuals 35 years old or younger with those 50 years old or older and with the overall population.
This study utilized non-parametric bootstrap to estimate geometric mean titer (GMT) for each person within these groups.
Shown in almost every case was that the group containing the younger people had 1.6-2.3 times higher GMT when compared to the older group, with the exception of the Beta variant following a single dose of vaccine. This may be due to most individual titers being near the lower detection threshold.
To predict vaccine effectiveness against the Delta variant, the authors used model fit analysis obtained from all ages neutralization data.
This analysis revealed that vaccine effectiveness decreases as age increases.
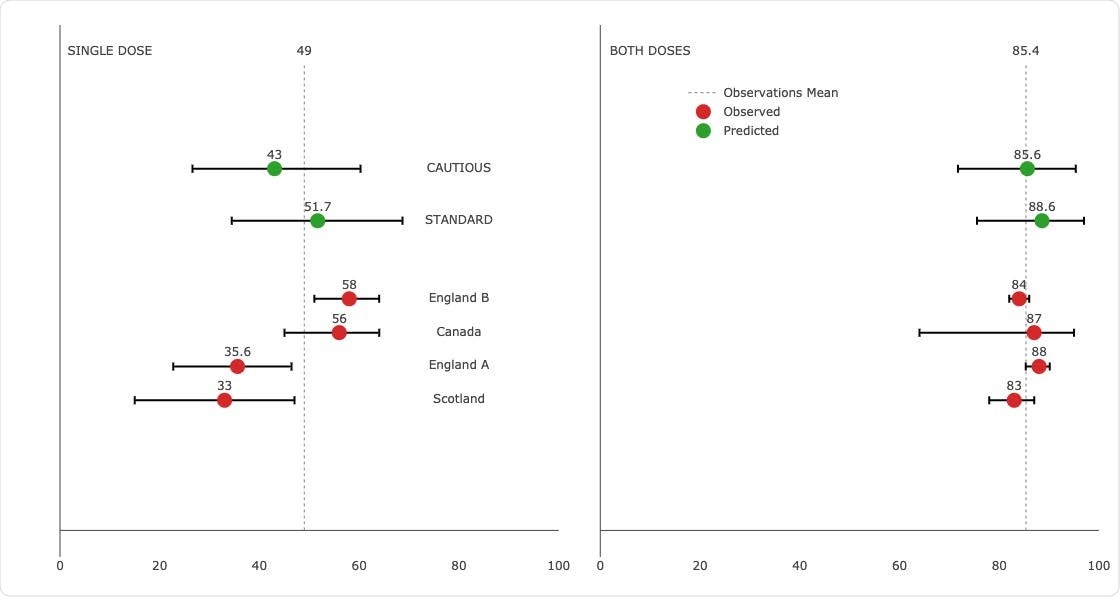
Predicting effectiveness against Delta. Estimates from effectiveness studies versus predictions from a model fit without Delta effectiveness observations. 95% prediction bands and weighted means of observations are shown.
Differences within the population
To challenge the assumptions set forth by previous research that titer distributions were representative of distributions in the vaccinated people within the population. The authors examined how violations of this assumption may have impacted their modeling due to population differences. They focussed on two studies to analyze population differences: Sheikh et al., 2021 and Wall et al. 2021.
To represent the “true population” in all the effectiveness studies concerning vaccinated people, the authors used data from Sheikh et al., 2021, although neutralization data was not included in this study. To resolve this problem, the authors analyzed data from Wall et al., 2021 as this paper investigated individual covariates for neutralization titers, such as age, BMI, and sex.
Titers from Wall et al., 2021 were utilized in combination with the age distribution from Sheikh et al., 2021, to represent the true population.
The authors state that there are uncertainties with both data sets and propose an approach to mitigate them. Following the author applying their approach to the data, it is suggested that the all-age effectiveness of the vaccinations would be at least 84.8%.
Predictions for the effectiveness of vaccination against the Delta variant
The authors applied their formulated framework to predict vaccine effectiveness against the Delta variant in the age groups 18-34 and 35-64. The predictions from a double dose of vaccine were particularly close.
The standard approach over-predicted the mean of effectiveness observations for all age groups by 3.2%. On the other hand, the cautious approach under-predicted the mean of effectiveness observations for all age groups by 0.2%.
Regarding a single dose, the standard approach over-predicted the observations mean for all age groups by 2.7% and the cautious approach under-predicted by 6%.
Implications
The framework proposed by the authors attempts to build a foundation towards the establishment of correlates of protection got the Comirnaty vaccinations.
The modeling utilized by the authors produced close predictions of the effectiveness of vaccination against the delta variant, especially following a double dose.
Sources:
- Sheikh, A., J. McMenamin, B. Taylor, and C. Robertson (2021, June). SARS-CoV-2 delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. The Lancet 397(10293), 2461–2462. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01358-1/fulltext
- Wall, E. C., M. Wu, R. Harvey, G. Kelly, et al. (2021, June). Neutralising antibody activity against SARS-CoV-2 VOCs b.1.617.2 and b.1.351 by BNT162b2 vaccination. The Lancet 397(10292), 2331–2333. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01290-3/fulltext
Journal reference: