Researchers in the United States and Germany have conducted a study showing that the Pfizer-BioNTech vaccine designed to protect against coronavirus disease 2019 (COVID-19) is effective against recently emerged variants of severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2).
The team – from the University of Texas Medical Branch in Galveston, Pfizer Vaccine Research and Development in New York and BioNTech in Mainz – showed that sera drawn from individuals who had received two doses of the BNT162b2 vaccine efficiently neutralized chimeric SARS-CoV-2 viruses bearing the spike proteins of the delta plus (delta-AY.1, delta-AY.2, delta-∆144), lambda (C.37), and B.1.1.519 variants of SARS-CoV-2.
The spike protein is the primary surface structure the virus uses to bind to and infect host cells and is the main target of neutralizing antibodies following vaccination or natural infection.
Pei-Yong Shi and colleagues say the findings support the ongoing BNT162b2 mass immunization strategy, together with the implementation of public health measures, to control currently circulating variants, minimize the emergence of new variants and bring an end to the COVID-19 pandemic.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Concerns surrounding the emergence of new variants
Since the SARS-CoV-2 outbreak first began in Wuhan, China, in late December 2019, the virus has accumulated mutations that increase transmissibility, replication, and escape from vaccine- or infection-induced immunity.
Many of the mutations have arisen in the viral spike protein, which mediates the initial stage of infection when it binds to the host cell receptor angiotensin-converting enzyme 2.
As the COVID-19 pandemic continues, it is critical to closely monitor recently emerged variants for their transmissibility, ability to cause severe disease, and potential to escape host immunity.
The BNT162b2 vaccine developed by Pfizer-BioNTech is a messenger RNA (mRNA)-based vaccine expressing the full-length SARS-CoV-2 spike protein. The vaccine has recently been approved for vaccination of individuals aged 16 years or older and has been authorized for emergency use among those aged 12 to 15 years.
“Although BNT162b2 mRNA encodes the original spike protein from the Wuhan isolate, the sera of those immunized with BNT162b2 can neutralize all tested variants, including the currently circulating delta variant,” writes Shi and colleagues.
“However, some variants are less efficiently neutralized than others,” they add.
What did the current study involve?
Shi and the team generated chimeric SARS-CoV-2 viruses bearing the spike proteins of distinct variants and tested their neutralization by the same panel of 20 sera samples drawn from trial participants 2 to 4 weeks after receiving the second dose of BNT162b2.
The SARS-CoV-2 panel included the spike from a wildtype viral strain (USA-WA1/2020) that was isolated in January 2020 and spikes from the following recently emerged variants.
- Strains referred to as “delta plus” that are closely related to the original delta variant. These included delta-AY.1 (first detected in India and spread to 32 countries); delta-AY.2 (first detected in the US and spread to 7 countries) and delta-∆144 (first detected in Vietnam and spread to 17 countries).
- The lambda (C.37) variant that was first detected in Peru and has spread to 46 countries, with high prevalence in South America.
- The B.1.1.519 lineage that emerged and became dominant in Mexico during the first months of 2021.
Each serum sample was tested simultaneously for its neutralizing activity against the wildtype and chimeric viral variants.
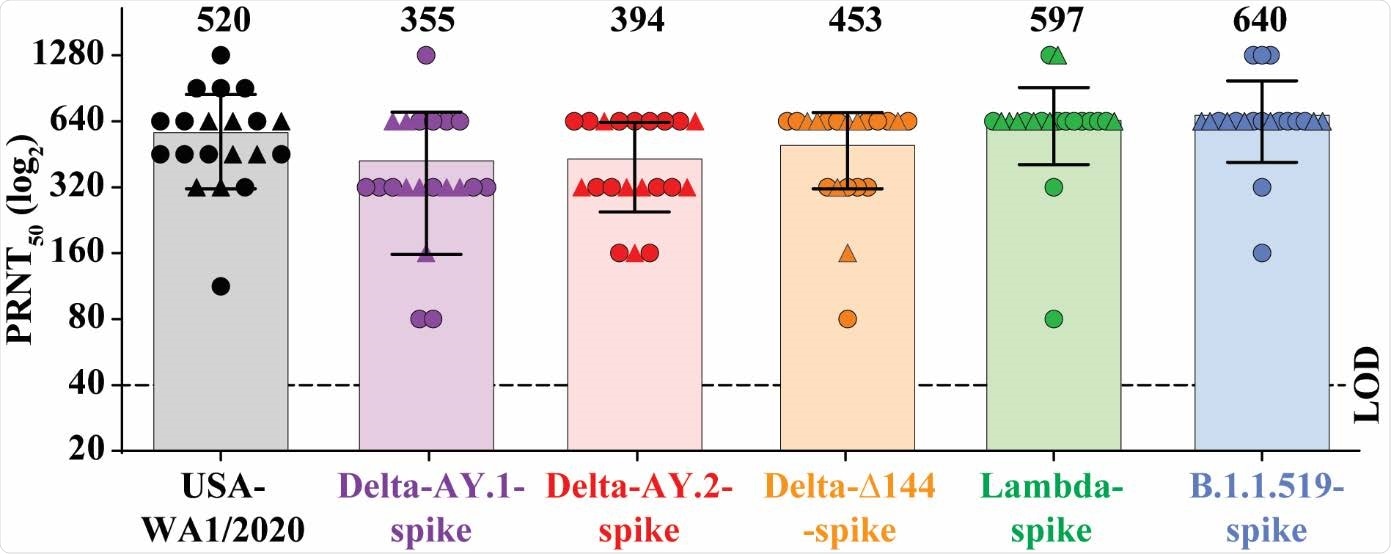
BNT162b2 vaccine-elicited neutralization of SARS-CoV-2 variants. The plot presents the PRNT50 titers of 20 human sera (drawn 2 or 4 weeks after two 30-μg doses of BNT162b2, spaced three weeks apart) against USA-WA1/2020 isolate and its chimeric viruses bearing distinct variant spikes. Serum samples obtained at 2 weeks or 4 weeks are represented by circles and triangles, respectively. Individual PRNT50 values are presented in Extended Data Table 1. Each data point represents the geometric mean PRNT50 against the indicated virus obtained with a serum specimen. The PRNT50 values were determined in duplicate assays, and the geometric means were calculated (n=20, pooled from two independent experiments). The bars and the numbers above the bars indicate geometric mean titers. The horizontal bars indicate 95% confidence intervals. The limit of detection (LOD) of the PRNT assay is 1:40 and indicated by a dashed line. Statistical analysis was performed using the two-tailed Wilcoxon matched-pairs signed-rank test. The statistical significances of the differences between geometric mean titers in the USA-WA1/2020 neutralization assay and in each variant virus neutralization assay with the same serum samples are as follows: P = 0.0264 for Delta-AY.1- spike; P < 0.030 for Delta-AY.2-spike; P = 0.255 for Delta-Δ144-spike; P = 0.193 for Lambdaspike and P = 0.007 for B.1.1.519-spike.
All sera neutralized all of the viruses
All sera neutralized both the wildtype and the mutant viruses with titers of 80 or higher.
Geometric mean neutralization titers against the wildtype, delta-AY.1, delta-AY.2, delta-∆144, lambda, and B.1.1.519 viruses were 520, 355, 394, 453, 597, and 640, respectively.
The results, therefore, indicate that compared with the wild-type virus, neutralization of the delta plus variants was only slightly reduced and neutralization of the lambda and B.1.1.519 variants was not reduced at all.
“Thus, BNT162b2 immune sera efficiently neutralized all tested viruses,” writes Shi and colleagues.
“The data in this paper support the ongoing BNT162b2 mass immunization strategy”
The researchers say that the susceptibility of the variants to neutralization indicates that antigenic change has not led to viral escape from vaccine-elicited neutralizing antibodies.
“The data in this paper support the ongoing BNT162b2 mass immunization strategy to control the variants and to minimize the emergence of new variants,” they write.
“Increasing the vaccination rate in the population, together with implementing public health measures, remains the primary means to end the COVID-19 pandemic,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Shi P, et al. BNT162b2-Elicited Neutralization of Delta Plus, Lambda, and Other Variants. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.09.13.460163, https://www.biorxiv.org/content/10.1101/2021.09.13.460163v1
- Peer reviewed and published scientific report.
Liu, Jianying, Yang Liu, Hongjie Xia, Jing Zou, Scott C. Weaver, Kena A. Swanson, Hui Cai, et al. 2022. “BNT162b2-Elicited Neutralization of Delta Plus, Lambda, Mu, B.1.1.519, and Theta SARS-CoV-2 Variants.” Npj Vaccines 7 (1): 1–4. https://doi.org/10.1038/s41541-022-00462-4. https://www.nature.com/articles/s41541-022-00462-4.