The rapid outbreak of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) caused the ongoing coronavirus disease 2019 (COVID-19) pandemic. Scientists suggested that this virus might have circulated in bat populations for decades before its spillover to humans. To date, this virus has infected over 233 million individuals and claimed more than 4.77 million lives.
SARS-CoV-2 was first reported in 2019 in Wuhan, China. Since then, this virus has been found to infect pets such as dogs, cats, ferrets, and minks. Researchers have also diagnosed SARS-CoV-2 infection in wild cougars, snow leopards, pumas, lions, and tigers. A high mortality rate has been reported in the mink farms in the Netherlands, Canada, Greece, Latvia, France, Denmark, the United States, and Sweden. Scientists are also concerned that mustelids, a family of carnivorous mammals, including weasels, badgers, otters, ferrets, martens, minks, and wolverines, among others, could become reservoirs for SARS-CoV-2.
A new study published on the medRxiv* preprint server presents a standardized survey instrument for evaluating risk factors associated with bidirectional zoonotic transmission of SARS-CoV-2 and pathogens. The instrument is particularly focused on settings where humans and animals share close contact.
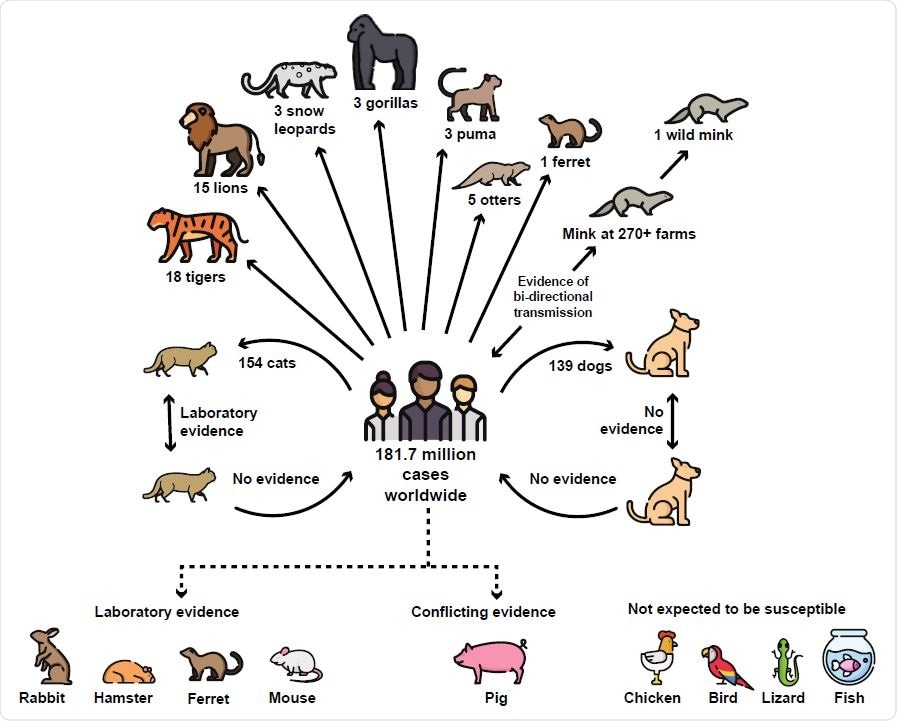
Global evidence to-date of CoV-2 transmission and susceptibility among common household pets and other animals. As of July 29, 2021, there have been 181.7 million human cases of COVID-19 globally (Johns Hopkins University, 2020). Multiple natural cases have been confirmed in animals since January 2020 in 31 countries worldwide. In addition, many CoV-2 outbreaks at mink farms have occurred in the Netherlands, Spain, Denmark, Italy, Sweden, Poland, Latvia, Greece, France, and Canada including secondary transmission from mink back to humans in Denmark (OIE, 2020; Oreshkova et al., 2020; USDA APHIS, 2020). In December 2020, the first free-ranging native wild animal, a wild mink, was confirmed with SARS-CoV-2 near a mink farm in the state of Utah, USA (ProMED, 2020). Laboratory evidence has confirmed that cats are infectious to other cats while there is no evidence of ongoing transmission in dogs (Halfmann et al., 2020). There is no evidence that household pets, including cats and dogs, act as ongoing reservoirs for transmission back to humans. While there have been no natural world cases of CoV-2 in rabbits, hamsters, or mice, laboratory studies have demonstrated these common household animals as susceptible to infection (Liu et al., 2020; Loeffler A, 2011). Multiple studies have concluded that pigs, chickens, other birds, reptiles, and fish are not expected to be susceptible to the virus (BondadReantaso M, 2020; Damas et al., 2020; Luan, Jin, Lu, & Zhang, 2020; Schlottau et al., 2020; Shi et al., 2020). Figure includes modified icons originally made by Freepik from www.flaticon.com.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
COVID-19 Human-Animal Interactions Survey (CHAIS)
The COVID-19 human-animal interactions working group (CHAI-WG) was established to develop the COVID-19 Human-Animal Interactions Survey (CHAIS). The main aim of CHAIS is to characterize human-animal interactions and understand risk factors related to two-way zoonotic transmission of CoV-2 and other zoonotic pathogens within settings where humans and animals are in close contact.
CHAIS measures four broad domains of transmission risk, namely, risk and intensity of infection among humans, spatial characteristics of shared spaces, human-animal interactions, and susceptibility of animals to infection, and the likelihood of onward spread.
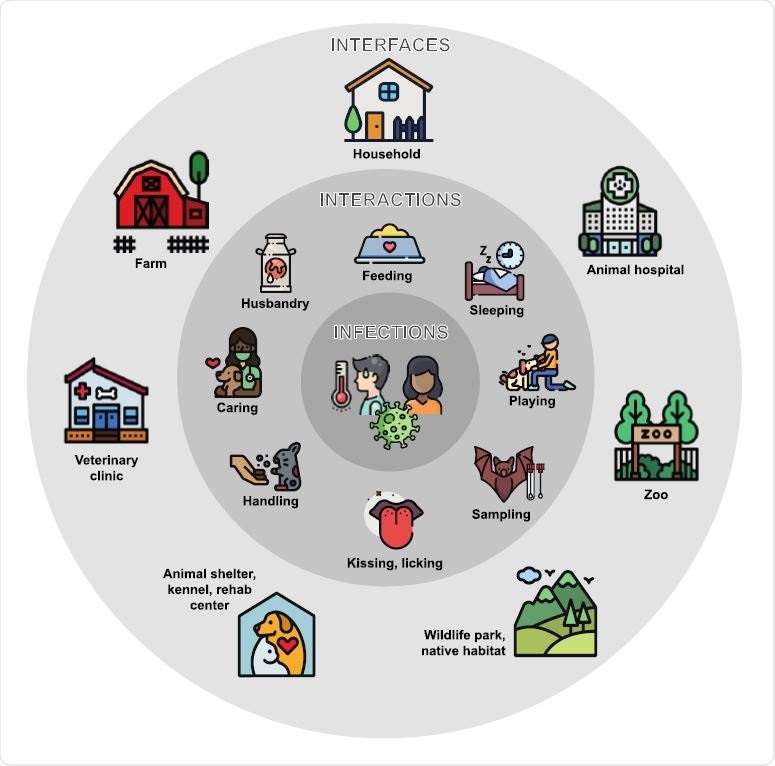
Common interfaces for human-animal interactions associated with reverse zoonotic transmission of respiratory pathogens
The CHAIS instrument should improve our understanding of pathogen exposure and transmission, provide a basis for predictive models, and provide an evidence base for public health guidance. The instrument comprises two versions - an extended version (E-CHAIS) detailing human-animal interactions and an abridged version (ACHAIS) including core questions addressing zoonotic and reverse zoonotic risk factors.
CHAIS is quite versatile in that it allows researchers to make minor amendments to questions to suit their studies of varying populations, temporality, cultural diversity, etc. In addition, they can select individual modules and use them in conjunction with others or select individual questions and cite the CHAIS instrument as the source.
The CHAIS instrument can be implemented in a broad range of research projects. In the studies that do not include a concurrent sampling of humans or animals, the instrument can be administered as an epidemiological survey to gather data from community members.
These data could be on human-animal interactions during the current pandemic or during other zoonotic outbreaks. CHAIS can also be used in surveillance and research studies that feature specimen collection from animals or both humans and animals. In these settings, the instrument can contextualize results in studies that include viral and/or antibody testing.
There are studies where samples are only collected from animals and in these studies, the CHAIS instrument may highlight factors associated with the animal’s exposure. CHAIS may also be used in studies with paired human-animal testing. It can help in measuring risk factors related to zoonotic and reverse zoonotic transmission.
CHAIS is a standard instrument for other zoonotic pathogens (besides SARS-CoV-2). There are important pathological differences between CoV-2 and other zoonotic pathogens. However, irrespective of those differences, the instrument is capable of measuring transmission risks that have broad epidemiologic applicability. This could provide crucial data on unique transmission pathways for these pathogens.
Limitations of CHAIS
There are a few limitations associated with the CHAIS instrument. CHAIS alone is not capable of serving as a robust assessment of the built environment and its effects on pathogen spread. For example, researchers might want to include questions related to ventilation systems, sanitary plumbing, etc. CHAIS is also limited in the extent to which it captures information about wildlife and wildlife-human interactions. It is also limited when focused on CoV-2 transmission during periods when other seasonal pathogens are operative. Finally, given the wide range of applications of CHAIS, biases in reporting among select populations may occur, and researchers should consider these biases when designing research studies, including the CHAIS instrument.
Conclusion
The CHAIS instrument is a standardized tool to analyze risks associated with the transmission of pathogens in environments where humans and animals are in close contact. It has broad applicability and importance owing to the gaps in behavioral, spatiotemporal, and biological factors underlying human-animal transmission.
The authors of this study have encouraged researchers to use this resource and provide feedback so that the questionnaire may be adapted based on available evidence. They also urged researchers to provide data for meta-analysis across studies so that we can further our understanding of the various factors that may lead to zoonotic and reverse zoonotic exposure and transmission of pathogens.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Gass, D.J. Jr. et al. (2021) A standardized instrument quantifying risk factors associated with bi-directional transmission of SARS-CoV-2 and other zoonotic pathogens: The COVID-19 Human-Animal Interactions Survey, (CHAIS). medRxiv 2021.09.21.21263227; doi: https://doi.org/10.1101/2021.09.21.21263227, https://www.medrxiv.org/content/10.1101/2021.09.21.21263227v1
- Peer reviewed and published scientific report.
Gass, Jonathon D., Kaitlin B. Waite, Nichola J. Hill, Kathryn R. Dalton, Kaitlin Sawatzki, Jonathan A. Runstadler, and Meghan F. Davis. 2022. “A Standardized Instrument Quantifying Risk Factors Associated with Bi-Directional Transmission of SARS-CoV-2 and Other Zoonotic Pathogens: The COVID-19 Human-Animal Interactions Survey (CHAIS).” One Health 15 (December): 100422. https://doi.org/10.1016/j.onehlt.2022.100422. https://www.sciencedirect.com/science/article/pii/S2352771422000544.