In less than one year, several highly efficacious messenger ribonucleic acid (mRNA) vaccines have been developed to protect against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The efficacy of the Moderna (mRNA1273) and Pfizer-BioNTech (BNT162b2) vaccines have been reported with 95% and 94.1% confidence limits.
Recent reports of vaccine effectiveness decreasing over time as a result of the emergence of new SARS-CoV-2 variants that appear to be resistant to neutralizing antibodies have increased concerns on the potential lack of durable immunity to vaccination. There have been recent reports stating the durability of antibody responses to the mRNA1273 vaccine; however, data on the durability of the BNT162b2 vaccine is lacking.
 Study: Durability of immune responses to the BNT162b2 mRNA vaccine. Image Credit: malazzama / Shutterstock.com
Study: Durability of immune responses to the BNT162b2 mRNA vaccine. Image Credit: malazzama / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
In a recent study published on the preprint server bioRxiv*, researchers analyzed the antibody responses to the homologous Wu strain, as well as several other SARS-CoV-2 variants including the Mu (B.1.621) variant. The authors of this study also assessed T-cell responses to the second dose of the BNT162b2 vaccine in six volunteers, six months (210 days) post initial vaccine dose.
What were the antibody and T-cell responses to the vaccines?
Out of the 56 volunteers in this study, the authors successfully collected blood samples from 46 participants 210 days post first vaccine dose. Between days 42 and 210, all 46 study participants exhibited a decline in their anti-spike binding immunoglobulin G (IgG) response, with two displaying a response below the limit of detection. In male participants, the decline in this response was 9.1-fold as compared to 6.3-fold in females.
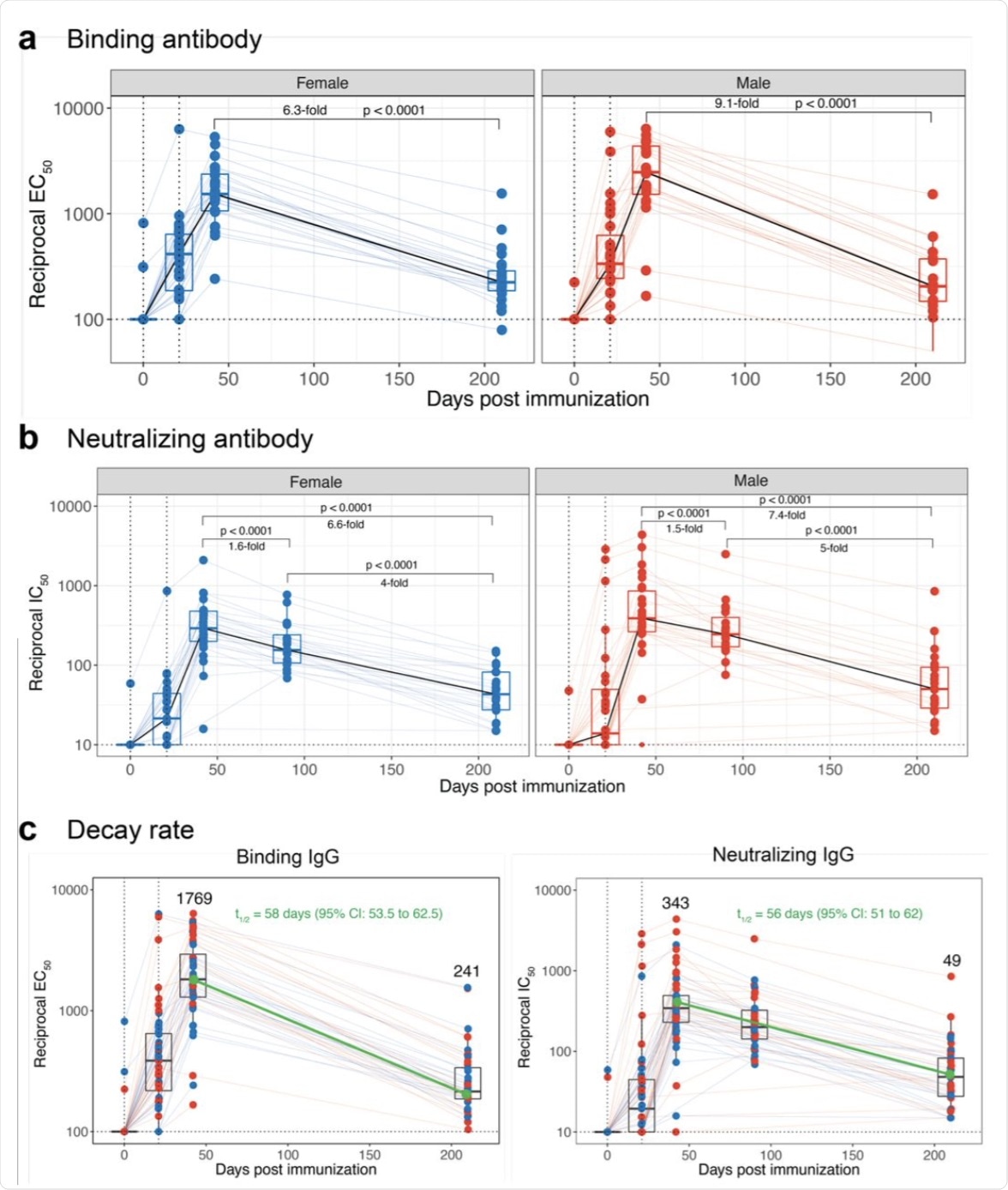 Durability of antibody following the Pfizer-BioNTech mRNA vaccination. a, Kinetics of anti-Spike binding IgG titers measured using ELISA (N = 46, 24 females and 22 males on day 210). Data of day 0 and 21 were obtained from our previously published study6. Day 42 samples were re-assayed with day 210 samples by ELISA. b, Kinetics of authentic live virus neutralizing antibody response against the homologous USA/WA1 strain (N = 46, 24 females and 22 males on day 210). Data of day 0, 21 and 90 were obtained from our previously published study6. Day 42 samples were re-assayed with day 210 samples by the FRNT assay. c, Kinetics of binding (left) and neutralizing (right) antibody responses of all participants. The dark green line indicates half-life estimated using the exponential decay model. In all the panels, each colored circle represents an individual. Red and blue circles indicate males and females, respectively. All statistical comparisons were non-parametric pair-wise comparisons performed using Wilcoxon’s matched-pairs signed-rank test.
Durability of antibody following the Pfizer-BioNTech mRNA vaccination. a, Kinetics of anti-Spike binding IgG titers measured using ELISA (N = 46, 24 females and 22 males on day 210). Data of day 0 and 21 were obtained from our previously published study6. Day 42 samples were re-assayed with day 210 samples by ELISA. b, Kinetics of authentic live virus neutralizing antibody response against the homologous USA/WA1 strain (N = 46, 24 females and 22 males on day 210). Data of day 0, 21 and 90 were obtained from our previously published study6. Day 42 samples were re-assayed with day 210 samples by the FRNT assay. c, Kinetics of binding (left) and neutralizing (right) antibody responses of all participants. The dark green line indicates half-life estimated using the exponential decay model. In all the panels, each colored circle represents an individual. Red and blue circles indicate males and females, respectively. All statistical comparisons were non-parametric pair-wise comparisons performed using Wilcoxon’s matched-pairs signed-rank test.
Consistent with these findings were the authentic live virus neutralization antibody titers against the homologous USA-WA1/2020 strain, which were 7-fold lower at six months relative to the three-week titers. Against the homologous strain, the durability of neutralizing antibodies titers was comparable to that which was observed with the mRNA1273 vaccine.
Using an exponential decay model, the authors calculated the decay rates of binding and neutralizing antibody responses. The half-lives of binding and neutralizing antibody responses were estimated to be 58 and 56 days, respectively. In contrast, the half-life of the live virus-neutralizing antibody response to the mRNA1273 vaccine was 68 days.
To measure the durability of memory T-cells, the authors measured the T-cell responses in 12 of the participants. When comparing day 210 to day 28, spike-specific CD4 T-cells expressing interferon- γ (IFN-γ) and tumor necrosis factor (TNF) were significantly downregulated,. However, there was no significant difference detected in interleukin-2 (IL-2+) CD4 T-cell response.
By day 210, the frequency of spike-specific circulating follicular T-helper cells expressing IL-21 and CD154 was also downregulated. Both of these cells are crucial for the generation of durable antibody responses.
Evaluation of cross-neutralization potential via live virus
The Focus Reduction Neutralization Test (FRNT50) geometric mean titers three weeks following the second dose of the vaccine were 302 for the WA1/2020 variant, 59 for the Beta variant, 175 for the Delta variant, 155 for the Gamma variant, and 130 for the Mu variant. The FRNT50 results at six months post second vaccine dose were 43 for WA1/2020, 26 for Beta, 25 for Delta, 44 for Gamma, and 32 for Mu.
Furthermore, on days 42 and 210, the efficiency of cross-neutralization of the variants of concern in serum was compared. On day 42, when compared to the homologous WA1 strain, the Beta variant showed the highest decline. This finding suggests this is the most difficult to neutralize variant, possibly due to mutations associated with immune escape from the vaccine or infection-induced antibodies.
The difference between the WA1 and the Beta variant was only 1.7-fold by 210, which suggests that cross-neutralizing antibodies were maintained. An undetectable neutralizing response was observed in 47% of patients, in response to the Delta variant, which was similar to the Beta variant. The neutralizing response to the Mu variant was also 2.3-fold lower when compared to the WA1 variant on day 42.
Implications
The current study finds that at six months post-vaccination with the BNT162b2 vaccine, there is a substantial decline of antibody responses and T-cell immunity to SARS-CoV-2. To enhance antibody titers and T-cell responses, a third booster immunization may be required due to a significant number of vaccines having neutralizing antibody titers below the detection limit.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Suthar, M. S., Arunachalam, P. S., Hu, M., et al. (2021). Durability of immune responses to the BNT162b2 mRNA vaccine. biorxiv. doi:10.1101/2021.09.30.462488. https://www.biorxiv.org/content/10.1101/2021.09.30.462488v1
- Peer reviewed and published scientific report.
Suthar, Mehul S., Prabhu S. Arunachalam, Mengyun Hu, Noah Reis, Meera Trisal, Olivia Raeber, Sharon Chinthrajah, et al. 2022. “Durability of Immune Responses to the BNT162b2 MRNA Vaccine.” Med 3 (1): 25–27. https://doi.org/10.1016/j.medj.2021.12.005. https://www.cell.com/med/fulltext/S2666-6340(21)00406-2.