The Pfizer-BioNTech BNT162b2 messenger ribonucleic acid (mRNA) COVID-19 vaccine and the Oxford-AstraZeneca ChAdOx1 nCoV-19 vaccine have been approved and widely administered as part of the national vaccination program in the United Kingdom. These vaccines were approved for use following Phase III randomized controlled trials (RCTs) that demonstrated clear evidence of the vaccine’s efficacy.
From vaccine rollout settings, observational studies confirmed vaccine effectiveness. However, to date, there have been no RCTs that directly compare the BNT162b2 and ChAdOx1 vaccines to estimate their relative efficacy against SARS-CoV-2 infection and disease in the same population.
Because the target populations and settings differ substantially from the clinical trials done prior to the vaccine authorization, the researchers undertook the current study to emulate such a trial on comparative vaccine efficacy using observational data. Such comparisons in observational data are difficult without the administration of both vaccines across the same population at the same time.
Health and social care workers (HCWs) were among the first group of people eligible for vaccination due to their high occupational risk of exposure to SARS-CoV-2, which causes COVID-19. While the HCW status becomes an important confounder on studying the effect of vaccination on COVID-19 outcomes, the researchers chose to study this group in isolation, thus eliminating the confounding factor.
Eliminating possible confounding and selection biases, as seen in comparisons of vaccinated and unvaccinated groups, the present study is the first to assess the effectiveness of the BNT162b2 and ChAdOx1 vaccines in a head-to-head comparison in either an experimental or observational setting.
About the study
The study cohort included 317,341 HCWs vaccinated between January 4 and February 28, 2021, who were registered with a general practice using the TPP SystmOne clinical information system in England. During the study period, both vaccines were widely used. Of the study cohort, 79.8% were vaccinated with BNT162b2 and 20.2% with ChAdOx1.
The vaccinated HCWs included in the study were between 18 and 64 years of age. These workers were not clinically extremely vulnerable.
With no way of anticipating the vaccine type to be associated with the health status of the workers, the HCWs were vaccinated with either BNT162b2 or ChAdOx1 administered as part of the national COVID-19 vaccine roll-out.
The researchers used a target-trial design to assess the effectiveness of ChAdOx1 as compared with BNT162b2 in the HCWs, including the second dose effectiveness using the OpenSAFELY-TPP linked primary care database. This database covers 24 million people registered at general practices, which is around 40% of England’s population.
The researchers defined three outcomes occurring within 20 weeks of vaccination in this study. These outcomes included a positive SARS-CoV-2 test, COVID-19 accident and emergency (A&E) attendance, and unplanned COVID-19 hospital admission.
Severe disease, such as intensive or critical care admission, and mortality events were minimal; therefore, the researchers did not investigate these outcomes fully.
The study results
The researchers presented the vaccinated participants of each vaccine versus the date. The graph showed that the BNT162b2 was on average administered earlier than ChAdOx1.
For comparative effectiveness, the researchers observed that post-vaccination, the ChAdOx1 versus BNT162b2 absolute risk difference per 1,000 people for a positive SARS-CoV-2 test, COVID-19 A&E attendances, and COVID-19 hospital admissions. They found that the outcomes were similar for those who received the BNT162b2 and ChAdOx1 COVID-19 vaccines.
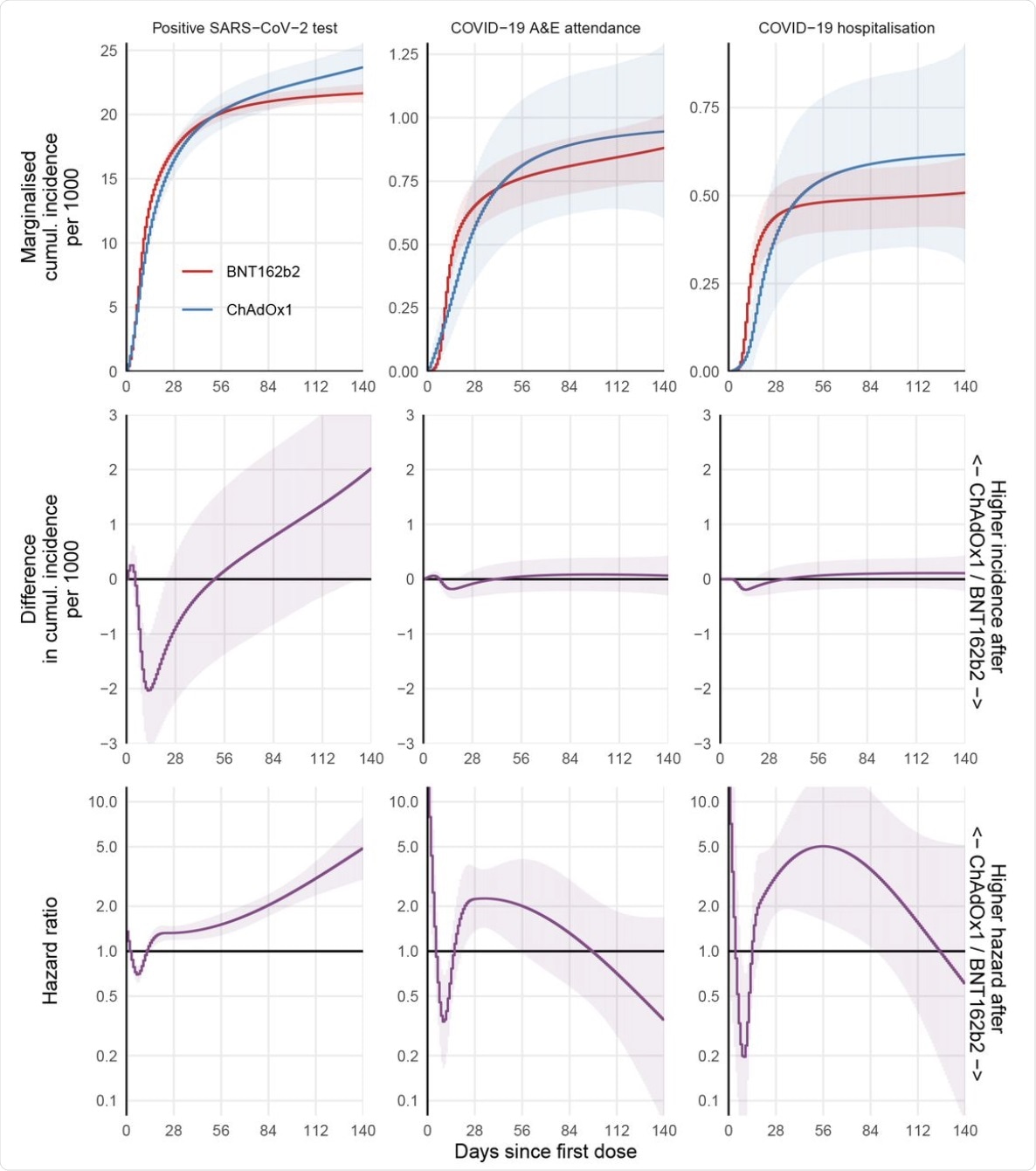 For each outcome based on the fully adjusted model, the marginal cumulative incidence for ChAdOx1 and BNT162b2, their difference, and the hazard ratio are shown. Models that assumed piecewise-constant hazards gave similar effect estimates (supplementary Figure S2). The models with less extensive confounder adjustment gave very similar estimates (supplementary Figure S1) suggesting that recipients of each vaccine were similar after accounting for differences in vaccine allocation over space and time (as did all models).
For each outcome based on the fully adjusted model, the marginal cumulative incidence for ChAdOx1 and BNT162b2, their difference, and the hazard ratio are shown. Models that assumed piecewise-constant hazards gave similar effect estimates (supplementary Figure S2). The models with less extensive confounder adjustment gave very similar estimates (supplementary Figure S1) suggesting that recipients of each vaccine were similar after accounting for differences in vaccine allocation over space and time (as did all models).
At 20 weeks, the researchers found a small advantage for the BNT162b2 vaccine as compared to the ChAdOx1 in terms of immunized people testing positive for SARS-CoV-2. However, the fact that the BNT162b2 recipients were more likely to receive their second dose sooner conferred a greater protective effect that is not accounted for in this study. The difference in the COVID-19 hospital attendance and admission events for the two vaccines was well below 1 event per 1,000 people in either direction.
The researchers cautioned that the results from the present study, which predominantly consisted of healthy HCWs, may not reflect the comparative effectiveness in more vulnerable groups such as the elderly or the immunosuppressed. Moreover, the dominant circulating variant during the time of this study was the Alpha variant, whereas the present one is the Delta variant, against which the vaccine’s effectiveness may vary. Particularly, the researchers noted that the potential immunological waning may differ.
Conclusion
Direct comparisons of first and second dose effectiveness between the ChAdOx1 and BNT162b2 COVID-19 vaccines showed similar incidence rates for infection and COVID-19-related hospital attendance and admission. This indicates strong protective effects for both vaccines. The researchers called for further studies to assess comparative effectiveness in newer, more prevalent variants and to assess longer-term effectiveness.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Hulme, W. J., Williamson, E. J., Green, A., et al. (2021). Comparative effectiveness of ChAdOx1 versus BNT162b2 COVID-19 vaccines in Health and Social Care workers in England: a cohort study using OpenSAFELY. medRxiv. doi:10.1101/2021.10.13.21264937. https://www.medrxiv.org/content/10.1101/2021.10.13.21264937v1
- Peer reviewed and published scientific report.
Hulme, William J., Elizabeth J. Williamson, Amelia C. A. Green, Krishnan Bhaskaran, Helen I. McDonald, Christopher T. Rentsch, Anna Schultze, et al. 2022. “Comparative Effectiveness of ChAdOx1 versus BNT162b2 Covid-19 Vaccines in Health and Social Care Workers in England: Cohort Study Using OpenSAFELY.” BMJ 378 (July): e068946. https://doi.org/10.1136/bmj-2021-068946. https://www.bmj.com/content/378/bmj-2021-068946.