The coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). One of the symptoms of COVID-19 is olfactory dysfunction, which is the reduced or distorted ability to smell during sniffing or eating.
 Study: Anosmia: High Prevalence, Plural Neuropathogenic Mechanisms, and Scarce Neurotropism of SARS-CoV-2? Image Credit: Design_Cells / Shutterstock.com
Study: Anosmia: High Prevalence, Plural Neuropathogenic Mechanisms, and Scarce Neurotropism of SARS-CoV-2? Image Credit: Design_Cells / Shutterstock.com
Olfactory dysfunctions
Olfactory dysfunctions are common disorders of the nose caused by nasal congestion, olfactory epithelium inflammation, infection, damage, or structural-functional abnormalities of the olfactory nerve, olfactory bulb, or other central nervous system (CNS) structures.
Olfactory dysfunctions in COVID-19 are different from the conventional sense of these disorders, as they occasionally appear before other symptoms and may even be the only symptoms of infection.
The incidence of smell and/or taste impairment in patients with COVID-19 varies. Studies report 5% to 98% prevalence, depending on areas, populations, SARS-CoV-2 variants, and methods of diagnosis. However, most studies report an olfactory dysfunction rate between 20% to 80%. The majority of COVID-19-related olfactory dysfunctions resolve in a few weeks; however, in some patients, these effects could persist long after the resolution of other COVID-19 symptoms.
Olfactory epithelium and SARS-CoV-2 susceptibility
The sense of smell or olfaction starts when airborne odor molecules bind to their receptors on the surface of the olfactory epithelium in the nasal cavity. The odorants elicit electrical signals that are transmitted through the olfactory nerves to the olfactory bulb in the brain.
The olfactory epithelium lines the nasal cavity near the entrance of the upper respiratory tract. This tissue helps in the early detection of important or potentially harmful odorants in the air. Importantly, the positioning of the olfactory epithelium also makes it vulnerable to pathogens or other types of damage.
The olfactory epithelium is like the respiratory epithelium that also lines the nasal cavity. However, careful examination reveals minute structural differences between these two tissues.
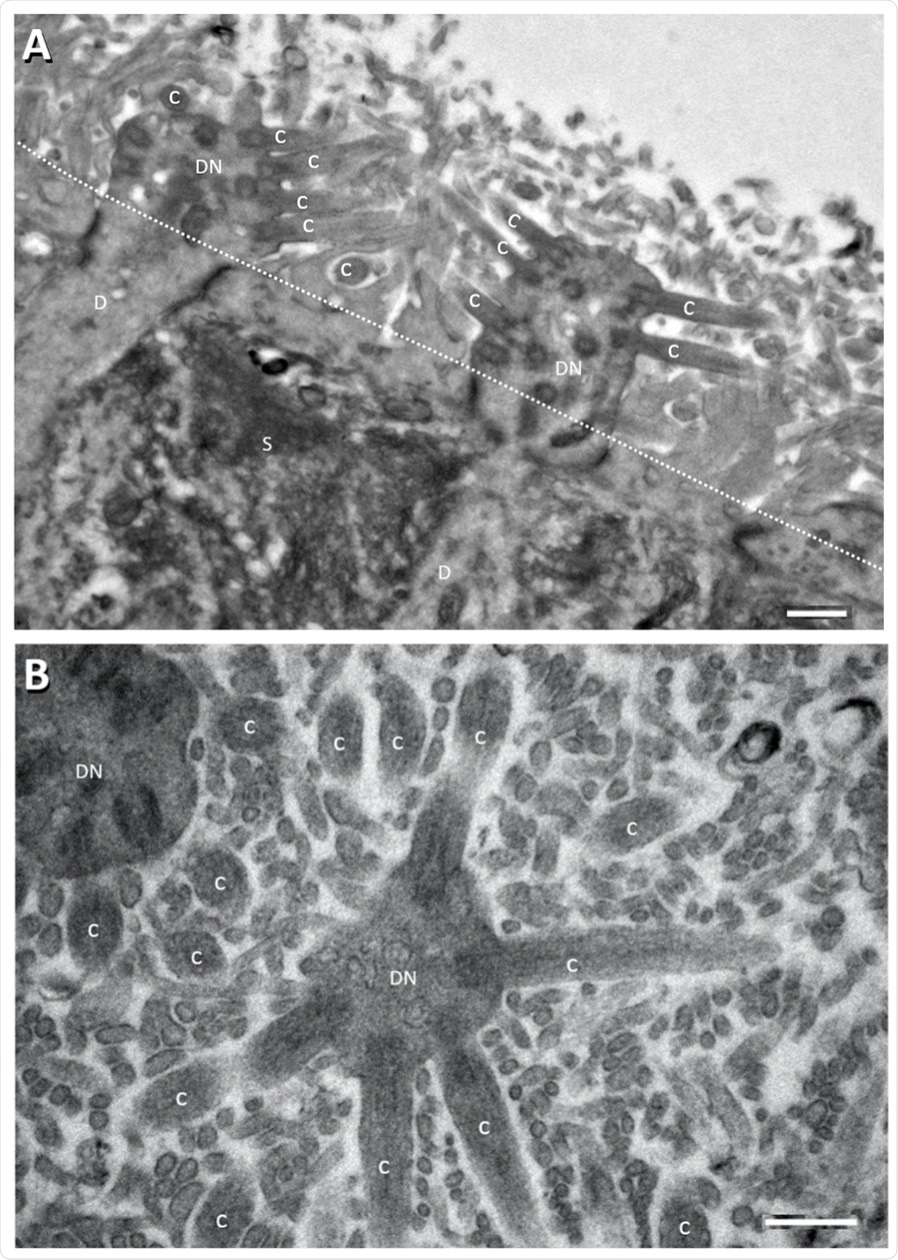 Electron micrographs showing perpendicular (A) and tangential/oblique section (B) of the apical part of the rat OE. Dotted line in panel A denotes sustentacular cell (S) apical surface from apical part of the rat OE. Dotted line in panel A denotes sustentacular cell (S) apical surface from which the long thin sustentacular-cell microvilli protrude into the nasal cavity for about 2–3 μm. which the long thin sustentacular-cell microvilli protrude into the nasal cavity for about 2–3 μm. ORN dendritic knobs (DN) and cilia (C) at apical ends of ORN dendrites (D) are mostly found ORN dendritic knobs (DN) and cilia (C) at apical ends of ORN dendrites (D) are mostly found among among the sustentacular microvilli (most of the unlabeled small profile structures in (B) and in area the sustentacular microvilli (most of the unlabeled small profile structures in (B) and in area above above the dotted line in (A). Human OE is similarly organized [52–54]. Scale bars = 0.5 μm. the dotted line in (A). Human OE is similarly organized [52–54]. Scale bars = 0.5 μm.
Electron micrographs showing perpendicular (A) and tangential/oblique section (B) of the apical part of the rat OE. Dotted line in panel A denotes sustentacular cell (S) apical surface from apical part of the rat OE. Dotted line in panel A denotes sustentacular cell (S) apical surface from which the long thin sustentacular-cell microvilli protrude into the nasal cavity for about 2–3 μm. which the long thin sustentacular-cell microvilli protrude into the nasal cavity for about 2–3 μm. ORN dendritic knobs (DN) and cilia (C) at apical ends of ORN dendrites (D) are mostly found ORN dendritic knobs (DN) and cilia (C) at apical ends of ORN dendrites (D) are mostly found among among the sustentacular microvilli (most of the unlabeled small profile structures in (B) and in area the sustentacular microvilli (most of the unlabeled small profile structures in (B) and in area above above the dotted line in (A). Human OE is similarly organized [52–54]. Scale bars = 0.5 μm. the dotted line in (A). Human OE is similarly organized [52–54]. Scale bars = 0.5 μm.
For example, there is a difference in expression of the angiotensin-converting enzyme 2 (ACE2) receptor, which is used by SARS-CoV-2 to gain entry into the host cell, between the olfactory epithelium and the respiratory epithelium. In fact, ACE2 expression is hundreds of times greater in the olfactory epithelium than in the respiratory epithelium. Furthermore, the olfactory epithelium has cellular microvilli that have increased cell surface area for binding or absorption.
Neuropathology of SARS-CoV-2
Viruses can preferentially bind to and enter nerves, thereby indicating their neurotropic nature. A majority of neurotropic viruses bind to receptors on the neurons. ACE2 is present on specific cell types within the olfactory epithelium and respiratory epithelium; however, there is minimal or no ACE2 on mature nerves of the olfactory system.
Several studies report the SARS-CoV-2 infection and/or pathology in various cells, tissues, and organs in human autopsy or biopsy samples, and in animal models. Moreover, some studies have also reported the presence of the SARS-CoV-2 spike protein in the olfactory epithelium and respiratory epithelium.
In one study, the biopsy from a patient with loss of smell three months after recovering from COVID-19 showed massive disruption and damage of the olfactory epithelium. SARS-CoV-2 was rarely detected in the cerebrospinal fluid (CSF) of COVID-19 patients; however, inflammation or immune reactions were commonly observed.
There is no direct evidence of SARS-CoV-2 infecting the brain. COVID-19 autopsies show extensive inflammation of the brain regions and injury to the olfactory bulb.
Mechanisms of COVID-19 neuropathogenesis
SARS-CoV-2 mainly infects olfactory sustentacular cells of the olfactory epithelium that express high levels of ACE2. Sustentacular cell infection and damage may lead to inflammation, immune reactions, as well as the release of cytokines and signaling which may cause olfactory dysfunctions and damage to the nerves of the olfactory system.
In the case of post-COVID-19 persistent olfactory dysfunctions, pathogenic mechanisms may include damage to certain cells, continuous inflammation, or chronic SARS-CoV-2 infection in the olfactory epithelium. Transport of pathogenic molecules from the olfactory epithelium to the olfactory bulb via the nerves may result in dysfunction and degeneration of nerves in the olfactory bulb. SARS-CoV-2 infection of cells in capillary blood vessels of the brain may compromise the blood-brain barrier and cause neuropathology and dysfunctions in various brain regions.
In patients with COVID-19 who have obvious nasal congestion, obstructed airflow through the nasal cavity would also adversely affect olfaction and intensify olfactory dysfunctions.
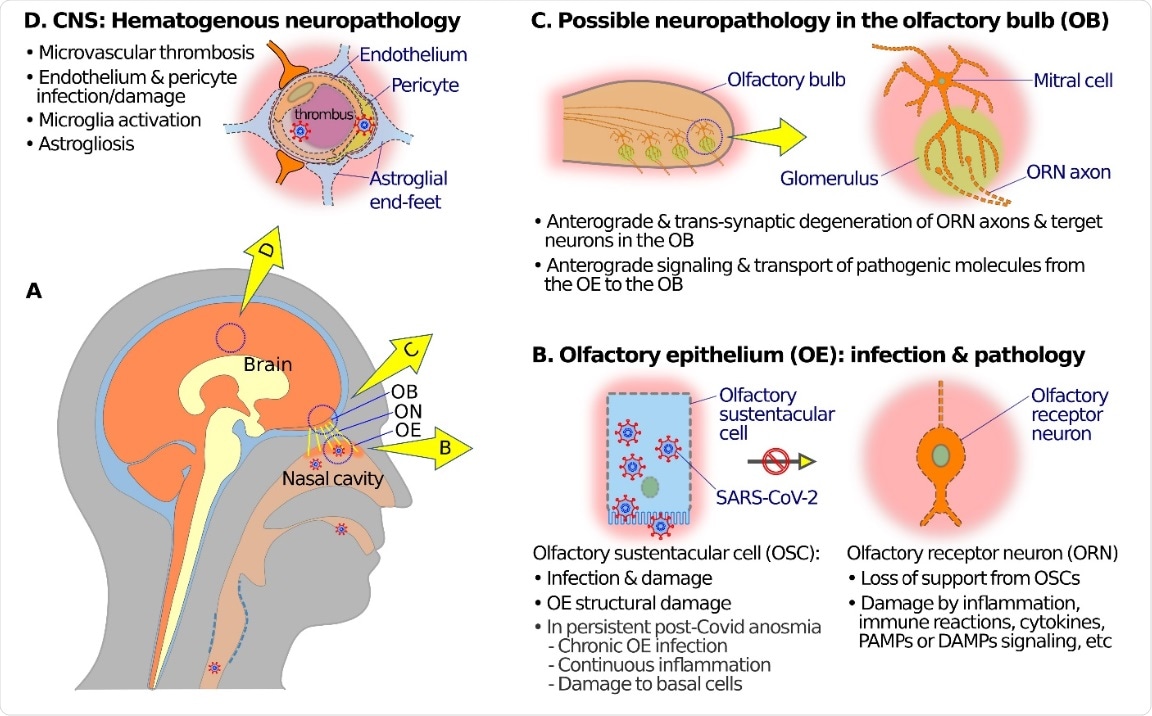 Schematic diagrams showing possible mechanisms of olfactory neuropathogenesis in COVID-19. (A) A schematic overview to illustrate relations among nasal cavity, olfactory epithelium (OE), olfactory nerve (ON), olfactory bulb (OB), and the brain (B) At the OE, SARS-CoV-2 mainly infects olfactory sustentacular cells (OSCs) that express high levels of SARS-CoV-2 receptor ACE2 on the luminal surface. Sustentacular cell infection and damage may lead to inflammation, SARS-CoV-2 receptor ACE2 on the luminal surface. Sustentacular cell infection and damage may lead to inflammation, immune reactions, release of cytokines, and signaling through pathogen-associated molecular patterns (PAMPs), damage- immune reactions, release of cytokines, and signaling through pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and pattern recognition receptors (PRRs) which in turn may cause dysfunctions associated molecular patterns (DAMPs), and pattern recognition receptors (PRRs) which in turn may cause dysfunctions (such as anosmia or hyposmia) and damage and/or anterograde degeneration of olfactory receptor neuronal cells (ORNs). In the case of post-COVID-19 persistent olfactory dysfunctions, pathogenic mechanisms may include damage of basal degeneration of neural structures in the OB. (D) SARS-CoV-2 infection of endothelial cells or pericytes, and microthrombi transport of pathogenic molecules from the OE to the OB along ORN axons may result in dysfunction and transsynaptic in capillary blood vessels, may compromise the blood–brain barrier, and give rise to hematogenous neuropathology and degeneration of neural structures in the OB. (D) SARS-CoV-2 infection of endothelial cells or pericytes, and microthrombi dysfunctions in various brain regions, including the OB. in capillary blood vessels, may compromise the blood–brain barrier, and give rise to hematogenous neuropathology and dysfunctions in various brain regions, including the OB.
Schematic diagrams showing possible mechanisms of olfactory neuropathogenesis in COVID-19. (A) A schematic overview to illustrate relations among nasal cavity, olfactory epithelium (OE), olfactory nerve (ON), olfactory bulb (OB), and the brain (B) At the OE, SARS-CoV-2 mainly infects olfactory sustentacular cells (OSCs) that express high levels of SARS-CoV-2 receptor ACE2 on the luminal surface. Sustentacular cell infection and damage may lead to inflammation, SARS-CoV-2 receptor ACE2 on the luminal surface. Sustentacular cell infection and damage may lead to inflammation, immune reactions, release of cytokines, and signaling through pathogen-associated molecular patterns (PAMPs), damage- immune reactions, release of cytokines, and signaling through pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and pattern recognition receptors (PRRs) which in turn may cause dysfunctions associated molecular patterns (DAMPs), and pattern recognition receptors (PRRs) which in turn may cause dysfunctions (such as anosmia or hyposmia) and damage and/or anterograde degeneration of olfactory receptor neuronal cells (ORNs). In the case of post-COVID-19 persistent olfactory dysfunctions, pathogenic mechanisms may include damage of basal degeneration of neural structures in the OB. (D) SARS-CoV-2 infection of endothelial cells or pericytes, and microthrombi transport of pathogenic molecules from the OE to the OB along ORN axons may result in dysfunction and transsynaptic in capillary blood vessels, may compromise the blood–brain barrier, and give rise to hematogenous neuropathology and degeneration of neural structures in the OB. (D) SARS-CoV-2 infection of endothelial cells or pericytes, and microthrombi dysfunctions in various brain regions, including the OB. in capillary blood vessels, may compromise the blood–brain barrier, and give rise to hematogenous neuropathology and dysfunctions in various brain regions, including the OB.
Long-lasting olfactory dysfunctions after COVID-19
The olfactory epithelium undergoes regular aging and self-replacement throughout life. Moreover, this tissue readily repairs or regenerates itself after damages.
The absent or slow recovery from COVID-19 olfactory dysfunctions in certain individuals implies severe or lasting damage to the olfactory epithelium by the virus. Another possible explanation is the persistent presence of SARS-CoV-2, chronic inflammation, and immune reactions, or increased cell death in the olfactory epithelium. Interestingly, chronic inflammation could switch the function of certain cells in the olfactory epithelium from regeneration to inflammatory signaling and immune cell proliferation.
Conclusion
There is no evidence of SARS-CoV-2 infecting the nerves. SARS-CoV-2 infection causes olfaction dysfunctions and damage, most likely through indirect means such as deprivation of support and inflammatory or immune reactions. These processes add to the pathogenic mechanisms for many of the COVID-19 neurological symptoms and complications.
Taken together, it is essential to investigate the causes and treatments of chronic SARS-CoV-2 infection of the olfactory epithelium and persistent post-COVID-19 olfactory dysfunctions in several COVID-19 convalescents.
Journal reference:
- Liang, F., & Wang, D. Y. (2021). COVID-19 Anosmia: High Prevalence, Plural Neuropathogenic Mechanisms, and Scarce Neurotropism of SARS-CoV-2? Viruses, 13(11), 2225. doi:10.3390/v13112225.