A team of international scientists has recently revealed that T cell responses induced by coronavirus disease 2019 (COVID-19) vaccines are able to cross-recognize different variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The study is currently available on the bioRxiv* preprint server.
With the progression of the COVID-19 pandemic, several SARS-CoV-2 variants with improved immunological fitness have emerged globally. The presence of multiple mutations in the spike protein has made these variants highly transmissible and probably more virulent. The most recently emerged variant is named “Omicron” by the World Health Organization (WHO). The variant has been designated by the WHO as a variant of concern (VOC) because of its highly increased transmissibility and ability to evade vaccine-induced humoral immunity.
Studies conducted in real-world pandemic setups have demonstrated that the majority of identified VOCs, including alpha, beta, gamma, delta, and omicron, have mutations in key spike epitopes that help develop resistance against antibody-mediated neutralization. However, insufficient information is available on memory T and B cell responses against different SARS-CoV-2 variants.
In the current study, the scientists have investigated cross-reactivity of COVID-19 vaccine-induced T cell responses against a panel of SARS-CoV-2 variants.
Study design
The study was conducted on 96 adults who had been immunized with mRNA-1273 (mRNA vaccine by Moderna), BNT162b2 (mRNA vaccine by Pfizer/BioNTech), Ad26.COV2.S (adenoviral vector vaccine by Johnson & Johnson), or NVX-CoV2373 (recombinant protein vaccine by Novavax) vaccines.
Blood samples were collected from the participants 2 weeks after the first dose, two weeks after the second dose, and 3.5 months and 5-6 months after the last vaccination.
The impact of different SARS-CoV-2 variants on vaccine-induced T cell response was assessed by mapping specific spike mutations of tested variants and comparing them with the wild-type virus (Wuhan strain).
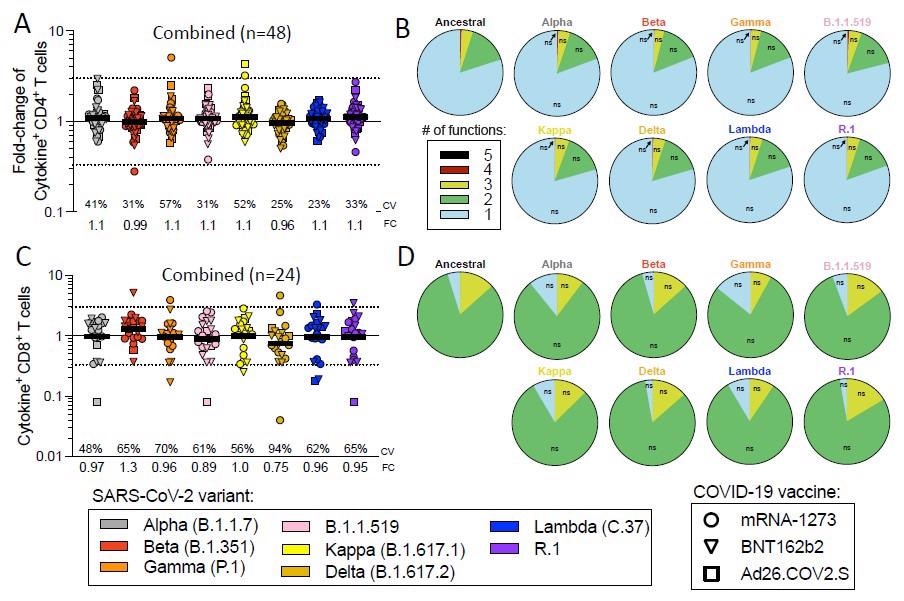
Impact of variant associated mutations on spike-specific cytokine responses in CD4+ and CD8+ T cells. Fully vaccinated COVID-19 vaccinees were assessed with variant spike MPs and the effect of mutations associated with each variant MP is expressed as relative (fold change variation) to the T cell reactivity detected with the ancestral strain MP. Results from COVID-19 mRNA-1273 (circles), BNT162b2 (triangles) and Ad26.COV2.S (squares) vaccinees are presented combined together. (A) Fold change values for cytokine+CD4+ T cells are calculated based on the sum of CD4+ T cells producing CD40L, IFNγ, TNFα, IL-2, or Granzyme B and (B) the functionality of the CD4+ T cell is calculated by looking at the different combinations of cytokines. (C) Fold change values for cytokine+CD8+ T cells are calculated based on the sum of CD8+ T cells producing IFNγ, TNFα, IL-2, or Granzyme B and (D) the functionality of the CD8+ T cell is calculated by looking at the different combinations of cytokines. Coefficients of variation (CV) for the variants are listed in each graph. Significance of fold change decreases for each variant was assessed by Wilcoxon Signed Rank T test compared to a hypothetical median of 1
Spike-specific T cell responses in fully vaccinated individuals
The study analyzed CD4+ and CD8+ T cell responses induced by each tested vaccine to the spike protein of a panel of SARS-CoV-2 variants, including alpha, beta, gamma, delta, B.1.1.519, kappa, lambda, and R.1 variants.
Considering all tested variants and vaccines, no significant reduction in T cell response was observed. Overall, the findings revealed that irrespective of vaccine types and variants tested, about 83% and 85% of CD4+ and CD8+ T cell responses, respectively, remain unaffected in fully vaccinated individuals.
Cross-reactivity of memory T cells
The ability of spike-specific memory B and T cells to cross-recognize different viral variants (alpha, beta, gamma, and delta) was assessed in the study. Overall, the findings revealed that memory CD4+ T cell recognition of all tested variants largely remains unaffected. However, regarding memory CD8+ T cells, a 1.9-fold reduction was observed against the delta variant.
The analysis of memory B cell recognition of alpha, gamma, and delta variants revealed a significant reduction. Similarly, a significant reduction of spike receptor-binding domain (RBD)-specific memory B cell recognition of alpha, beta, gamma, and delta variants was observed. In addition, a 2.4-fold, 4.5-fold, 3.8-fold, and 3.4-fold reduction in neutralizing titers against alpha, beta, gamma, and delta variants, respectively, was also observed.
Impact of spike mutations on T cell epitopes
The study included bioinformatic analyses to predict the impact of variant-specific mutations on CD4+ and CD8+ T cell epitopes spanning the entire spike protein. The findings revealed that 91% and 94% of CD4+ and CD8+ T cell epitopes, respectively, are fully conserved across a panel of variants, including alpha, beta, gamma, delta, epsilon, eta, iota, kappa, lambda, and R1. However, for the omicron variant, the fractions of fully conserved CD4+ and CD8+ epitopes reduced to 72% and 86%, respectively. This could be because of the high numbers of mutations present in the omicron spike.
Impact of omicron mutations on T cell response
The impact of omicron mutations on T cell response was assessed in individuals who had received the COVID-19 vaccine 5 – 6 months before. The findings revealed that the majority of CD4+ and CD8+ T cell responses are preserved against the omicron variant, with some CD8+ T cell responses showing a reduction.
Furthermore, epitope repertoire analysis revealed that about 80% of T cell responses are associated with fully conserved epitopes in the omicron variant. Each vaccinated participant recognized about 10 and 11 spike-specific CD8+ and CD4+ T cell epitopes, respectively.
Study significance
The study reveals that the majority of vaccine-induced T cell responses are able to cross-recognize a range of SARS-CoV-2 variants, including delta and omicron. Despite the reduction in vaccine-induced neutralizing titers, such highly conserved T cell immunity is expected to play a vital role as a second line of defense against emerging viral variants.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources