A recent study on vaccine effectiveness showed that a complete primary immunization with CoronaVac effectively protects against severe coronavirus disease 2019 (COVID-19) for children and adolescents between 6 and 16 years of age – consistent with the results from phase 2 clinical trials. The paper is currently available on the Preprints with The Lancet* server while it undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
While the pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is still ongoing, the debate is still not set on the benefits of vaccinating children against it. More specifically, the cost-benefit analysis is not straightforward, especially if we take into account global COVID-19 vaccination targets and inequities in vaccine access.
However, literature shows certain benefits, as vaccination can prevent severe COVID-19 cases and potential deaths among children with underlying health conditions. Furthermore, the vaccination may prevent long-term consequences of SARS-CoV-2 infection, including multisystem inflammatory syndrome in children (MIS-C) and long-COVID.
In addition, vaccination can also reduce transmission to other individuals (regardless of age) and, by alleviating community transmission, may aid in reducing the need for non-pharmaceutical interventions – especially school closures, quarantines and lockdowns.
Previous data have suggested that an inactivated SARS-CoV-2 vaccine (also known as CoronaVac) is safe and immunogenic for children, and induced well-documented humoral responses in this age group (i.e., up to 17 years of age).
This paper, first-authored by Dr. Alejandro Jara from the Chilean Ministry of Health and Pontificia Universidad Católica de Chile, provides estimates of the effectiveness of CoronaVac in children and adolescents between 6 and 16 years of age in a countrywide mass vaccination campaign for preventing COVID-19 infection, hospitalization, and admission to the intensive care unit (ICU).
Appraising a national cohort
The study was conducted in Chile between June 27, 2021, and January 12, 2022. The authors have used a large prospective national cohort that included approximately two million children and adolescents.
The main goal was to compare the risk of individuals treated with a complete primary immunization schedule (two doses, 28 days apart) with the risk of unvaccinated individuals during the follow-up period.
For that purpose, the authors have used specific inverse probability-weighted survival regression models to appraise hazard ratios of complete immunization over the unvaccinated status, accounting for the difference in vaccination exposure and adjusting for relevant confounders.
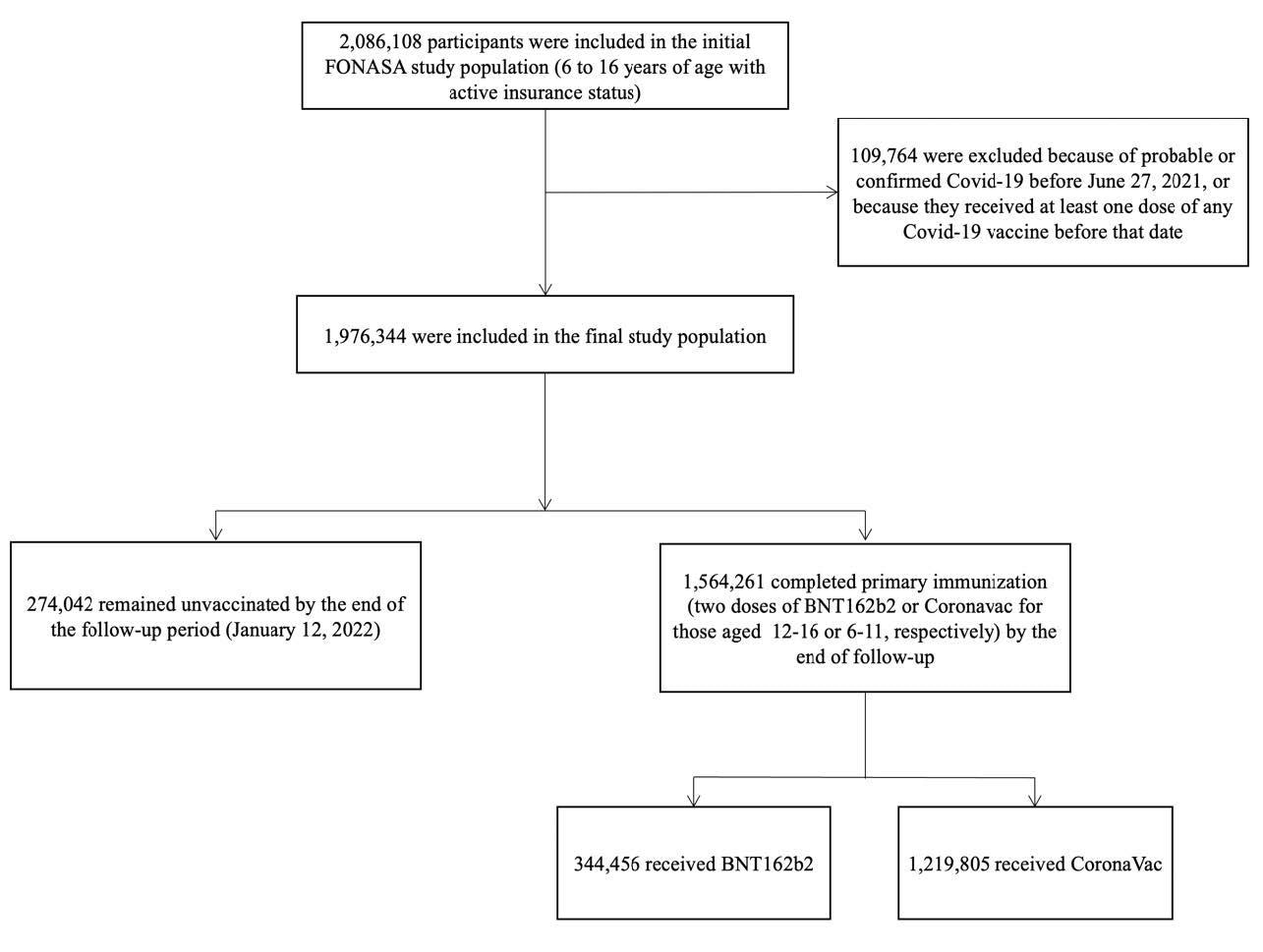
Study participants and cohort eligibility, June 27, 2021, to January 12, 2022. Participants were between 6 to16 years of age, affiliated to the Fondo Nacional de Salud (FONASA), the public national healthcare system, and vaccinated with a complete primary immunization (2 doses 28 days apart) with CoronaVac (6-16 years) or BNT162b2 (12-16 years) Covid-19 vaccines between June 27, 2021, and January 12, 2022, or not receiving any Covid-19 vaccination. We excluded individuals who had probable or confirmed coronavirus disease 2019 (Covid-19) according to reverse-transcription polymerase-chain reaction assay for SARS-Cov-2 or antigen test before June 27, 2021.
The effectiveness of full immunization
In a nutshell, the obtained vaccine effectiveness estimates imply that a complete primary immunization schedule provides somewhat effective protection against severe forms of COVID-19 for children aged between 6 and 16 years of age.
More specifically, for children and adolescents that underwent a full primary immunization with CoronaVac, the adjusted vaccine effectiveness was 74.5%, 91.0%, and 93.8% for COVID-19 development, hospitalization, and ICU admission, respectively.
Moreover, a subgroup of children aged between 6 and 11 years had adjusted vaccine effectiveness of 75.8% to prevent COVID-19 and 77.9% from avoiding hospitalization. These percentages were much higher for adolescents aged 12 to 16 years, reaching 93.5% for preventing COVID-19 hospitalizations.
Reducing COVID-19 disease burden
The main strengths of this study include a large cohort and real-world estimates that examine one of the most pervasively used COVID-19 vaccines around the world. Conversely, the main limitations are potential selection and misclassification biases and the absence of data needed to estimate vaccine effectiveness for specific variants of concern adequately.
“There is increasing evidence that vaccinating children and adolescents may significantly reduce the disease burden of COVID-19”, say study authors. “We hope our estimates will help inform this ongoing debate and support urgent decision-making globally in responding to COVID-19”, they add.
In any case, such vaccine effectiveness estimation endeavors are crucial in reflecting real-world challenges of vaccination rollout (such as logistics, cold chains and scheduling issues), and include a more diverse population than is possible with controlled trials. More studies akin to this one will be needed as we go forward.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources