
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The subsequent emergence of SARS-CoV-2 variants with improved transmissibility, immune evasion, and viral fitness is responsible for the ongoing coronavirus disease 2019 (COVID-19) pandemic. The most recently discovered SARS-CoV-2 variant of concern (VOC), Omicron, has the highest known genomic divergence from Wuhan-Hu-1, the SARS-CoV-2 ancestral strain. Omicron has three sublineages named BA.3, BA.2, and BA.1. Omicron BA.1 emerged in late 2021 and displaced the globally dominant Delta variant. Further, the Omicron BA.2 sublineage gradually displaced the worldwide dominant BA.1.
The current COVID-19 vaccines are based on the SARS-CoV-2 spike (S), receptor-binding domain (RBD), and inactivated full virus. In addition, the COVID-19 vaccines utilize various delivery techniques such as: 1) lipid-encapsulated prefusion-stabilized SARS-CoV-2 S-encoding messenger ribonucleic acid (mRNA)-based vaccines like Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273), 2) viral vectored S-encoding vaccines like Janssen (Ad26.COV2.S), AstraZeneca (AZD1222), and Sputnik V, 3) prefusion-stabilized S protein-subunit vaccine containing a saponin-based matrix M adjuvant like Novavax (NVX-CoV2373), and 4) perfusion -stabilized vaccine formulated with inactivated virions such as BBIBP-CorV.
About the study
In the present study, the researchers evaluated the immune evasion linked with the array of S mutations seen in SARS-CoV-2 BA.2 and BA.1 sublineages. For this, the team compared the plasma neutralizing activity induced by SARS-CoV-2 infection or seven COVID-19 vaccines (BNT162b2, mRNA-1273, Ad26.COV2.S, AZD1222, Sputnik V, NVX-CoV2373, and BBIBP-CorV). The scientists determined the invasion of vesicular stomatitis virus (VSV) pseudotyped with Wuhan-Hu-1 S bearing the BA.2, BA.1, or D614G mutations into VeroE6 cells stably producing transmembrane serine protease 2 (TMPRSS2) in convalescent or vaccinee plasma.
Depending on the time of infection, convalescent plasma was taken from people infected by a Washington-1-like SARS-CoV-2 variant. Further, neutralizing geometric mean titer (GMT) of plasma was determined for the SARS-CoV-2-infected and COVID-19 vaccinated subjects.
Results
The results indicated that GMT against G614 VSV S pseudovirus in SARS-CoV-2 convalescent plasma was 162. However, just seven and six out of 14 people exhibited detectable yet weak neutralizing activity against BA.2 and BA.1, respectively. By contrast, patients who got two shots of COVID-19 mRNA vaccines at three to four weeks intervals responded better against Omicron sublineages than those who had previously been infected. The BA.2, BA.1, and G614 S VSV neutralizing GMTs in participants vaccinated with two Moderna vaccine doses were higher than the Pfizer two-dose vaccinated subjects. The GMT dampening against BA.2 was lower than the Omicron BA.1 sublineage in the SARS-CoV-2 mRNA vaccinated individuals.
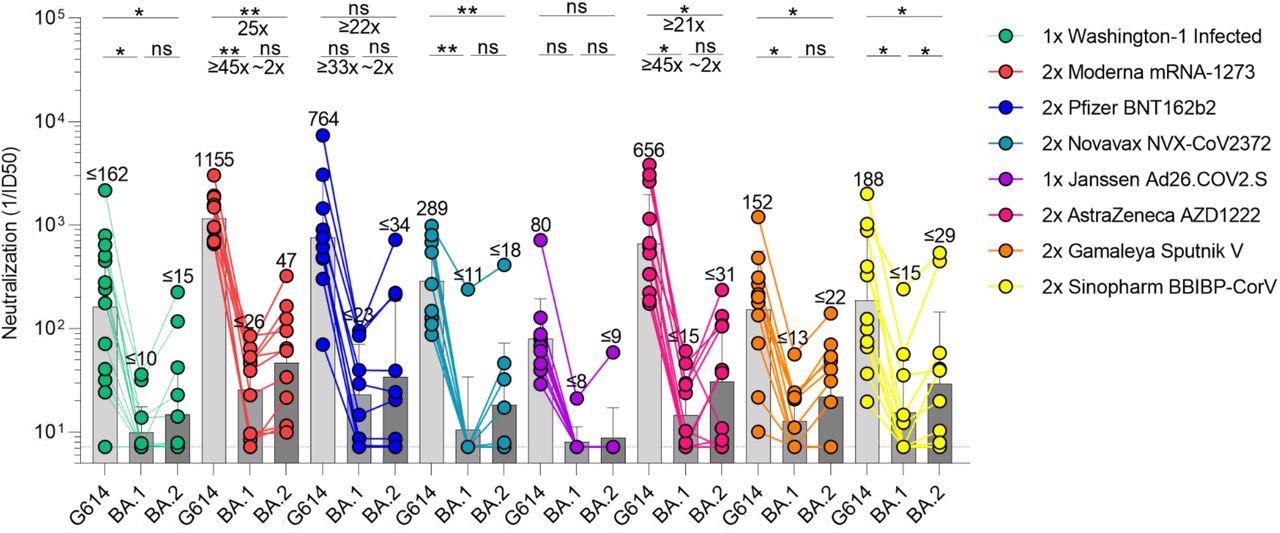
SARS-CoV-2 Omicron BA.1 and BA.2 evade human plasma neutralizing antibodies elicited by infection or primary vaccine series. Plasma neutralizing antibody titers elicited by primary COVID-19 vaccination determined using SARS-CoV-2 spike VSV pseudotypes using VeroE6-TMPRSS2 as target cells. Individual points are representative geometric mean titers from two independent experiments consisting of two replicates each. Bars represent geometric means and error bars represent geometric standard deviations for each group. Statistical significance between groups of paired data was determined by Wilcoxon rank test and *p< 0.05, **p< 0.01, ***p< 0.001, ****p< 0.0001.
In the Novavax two-dose vaccinated subjects, the GMT against BA.2 was more than BA.1. The single dose of Ad26.COV2.S vaccine exhibited a GMT of 80 against G614 S VSV, whereas just one out of 10 participants demonstrated measurable plasma neutralizing activity against both the Omicron sublineages. The GMT exhibited by two-dose AZD1222 vaccinated subjects at a four-week gap for BA.2 was higher than BA.1. In addition, these subjects demonstrated ≥21-time and ≥45-time degree reduction in GMT against BA.2 and BA.1, respectively.
Among the Sputnik, V two-dose vaccinated participants, the GMT against G614 S VSV was 153, and seven out of 12 subjects had measurable neutralizing activity against the Omicron BA.1 and BA.2 sublineages. These individuals exhibited GMT drops of ≥7 and ≥12 times for the BA.2 and BA.1 sublineages, respectively. BBIBP-CorV two-dose vaccinated people had a GMT of 188 against G614 S VSV, and eight and five out of 12 participants demonstrated measurable neutralizing activity against Omicron BA.2 (≥6-time GMT fall) and BA.1 (≥12-time GMT decrease) sublineages, respectively.
The mRNA vaccinated subjects who received a third mRNA-based booster vaccine dose demonstrated substantially higher GMT to the G614, BA.2, and BA.2 strains. The previously observed GMT drop against the Omicron BA.2 and BA.1 sublineages in these participants also improved dramatically. The Ad26.COV2.S single-dose vaccinated subjects who received a homologous or heterologous (BNT162b2) boost also demonstrated a markedly heightened GMT against the three tested SARS-CoV-2 variants. Their GMT drop against the two Omicron sublineages also enhanced significantly.
The two-dose AZD1222 vaccinated individuals who received a boost with the mRNA-based COVID-19 vaccine after six months demonstrated significantly high GMT against the BA.2, BA.1, and G614 variants. The reductions in GMT against BA.2 and BA.1 sublineages in this group were similar to the three-dose mRNA vaccinated participants. People who received a two-dose Sputnik V vaccine when boosted with BNT162b2 or AZD1222 after nine months exhibited substantially elevated GMTs against the three evaluated SARS-CoV-2 variants. The reductions in GMT against the Omicron BA.2 and BA.1 variants among these individuals were dramatically improved by the booster vaccination.
Conclusions
The study findings demonstrated that the vast number of BA.2 and BA.1 spike mutations significantly reduce plasma neutralizing activity evoked by the seven COVID-19 vaccines or SARS-CoV-2 infection. The Omicron BA.2 cross-neutralization was consistently more potent than BA.1, regardless of the number of doses or vaccine platform. Although COVID-19 mRNA-based vaccines elicited the highest levels of Omicron BA.2 and BA.1 plasma neutralizing ability, a booster vaccine centered on the SARS-CoV-2 Wuhan-Hu-1 S sequence significantly raised neutralizing antibody breadth and titers against BA.2 and BA.1 among all vaccines tested.
To summarize, the current findings imply that although the SARS-CoV-2 Omicron BA.2 and BA.1 sublineages elude polyclonal neutralizing antibody reactions, existing vaccine boosting strategies might be adequate to safeguard against Omicron-induced illness.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Omicron BA.1 and BA.2 neutralizing activity elicited by a comprehensive panel of human vaccines, John E. Bowen, Kaitlin R. Sprouse, Alexandra C. Walls, Ignacio G. Mazzitelli, Jennifer K. Logue, Nicholas M. Franko, Kumail Ahmed, Asefa Shariq, Elisabetta Cameroni, Andrea Gori, Alessandra Bandera, Christine M. Posavad, Jennifer M. Dan, Zeli Zhang, Daniela Weiskopf, Alessandro Sette, Shane Crotty, Najeeha Talat Iqbal, Davide Corti, Jorge Geffner, Renata Grifantini, Helen Y. Chu, David Veesler, bioRxiv, 2022.03.15.484542; doi: https://doi.org/10.1101/2022.03.15.484542, https://www.biorxiv.org/content/10.1101/2022.03.15.484542v1
- Peer reviewed and published scientific report.
Bowen, John E., Amin Addetia, Ha V. Dang, Cameron Stewart, Jack T. Brown, William K. Sharkey, Kaitlin R. Sprouse, et al. 2022. “Omicron Spike Function and Neutralizing Activity Elicited by a Comprehensive Panel of Vaccines.” Science, July. https://doi.org/10.1126/science.abq0203. https://www.science.org/doi/10.1126/science.abq0203.