How much energy do your gut microbes really provide? New research finds that what you eat, especially fiber, matters more than your microbiome’s makeup for fueling your body with beneficial fermentation products.
Theory: Quantifying the varying harvest of fermentation products from the human gut microbiota
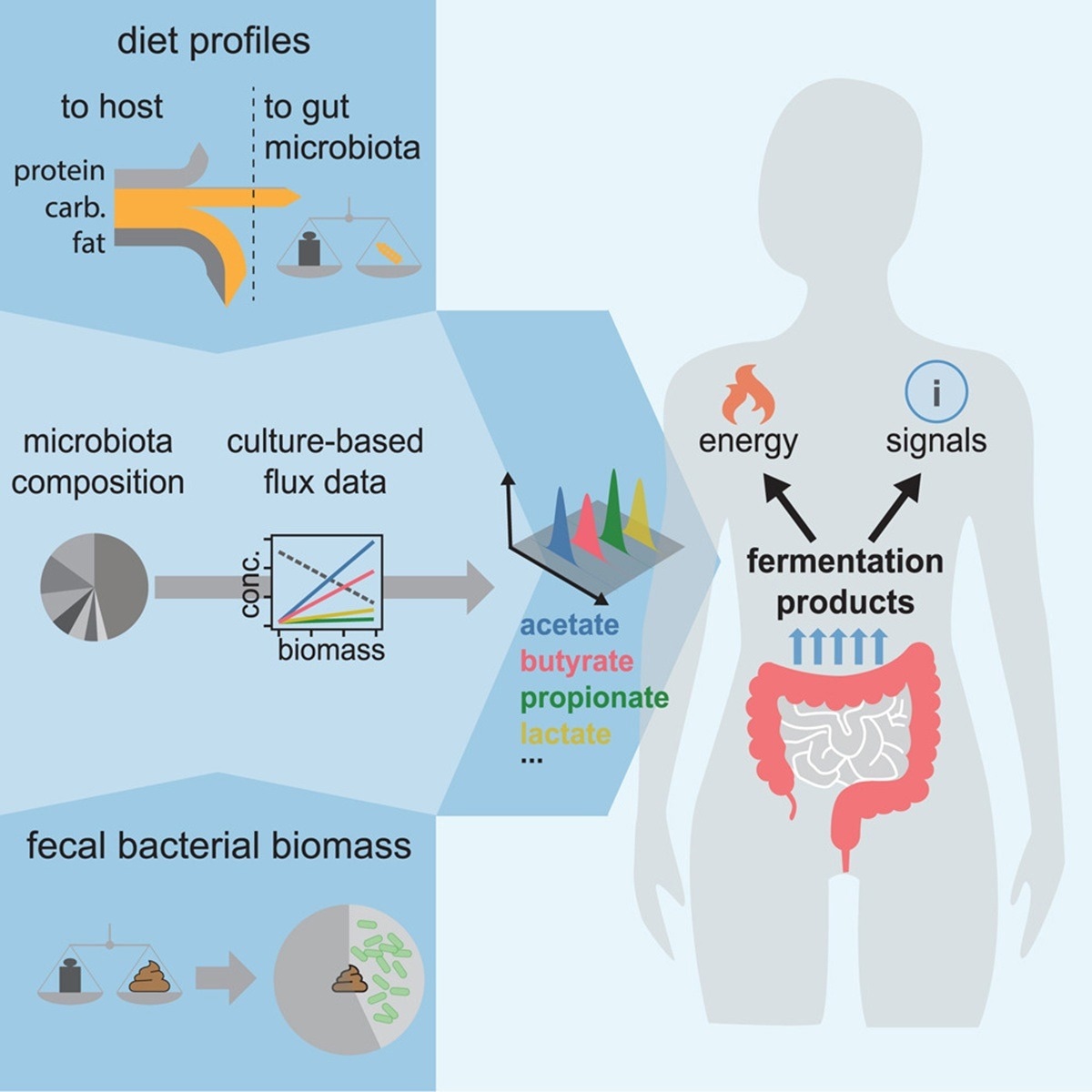
In a recent study published in the journal Cell, a group of researchers determined, with systems-level precision, how variations in diet and gut microbiota composition alter the quantity and composition of fermentation products absorbed by humans.
However, the authors note that the study's approach has several limitations, including its reliance on a static microbiome composition, limited explicit modeling of cross-feeding interactions, and a focus on major fermentation metabolites while excluding less abundant compounds.
Background
Imagine colon bacteria paying part of your grocery bill: in fiber-rich diets, they can supply up to one-tenth of daily calories. These anaerobes ferment otherwise indigestible complex carbohydrates into short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate that nourish colonocytes, modulate immunity, and even influence brain signaling. The proportion harvested depends on how much microbiota-available carbohydrates (MACs) reach the large intestine and which bacterial guilds dominate the ecosystem. Yet quantitative estimates of this flux in humans remain contentious, hampered by snapshot stool measurements and limited integration of dietary, physiological, and metagenomic data; therefore, systems-level accounting is needed.
About the study
Researchers first isolated 22 prevalent gut bacterial species from healthy adults and revived them from −80°C glycerol stocks under anaerobic workbench conditions. Seed cultures in four different types of media (YCA, BHIS, γ, and ε) were serially diluted to ensure exponential growth before experimental inoculations at an optical density at 600 nanometers (OD600) of ≈ 0.02. Growth was monitored by spectrophotometry, and four to six culture samples were filtered during the logarithmic phase to measure substrate uptake and metabolite release.
Metabolite concentrations were quantified by isocratic high-performance liquid chromatography (HPLC) with refractive-index detection using an ion-exchange column, 0.4 milliliter-per-minute flow, and 2.5 millimolar sulfuric acid mobile phase. Per-biomass rates of carbohydrate consumption and SCFA excretion were calculated from linear relationships between optical density and chromatogram peak areas. These rates were merged with genus-level relative abundances in 219 metagenomes and with dietary, fecal-mass, and physiological data to compute daily bacterial biomass production and fermentation-product fluxes. Two complementary estimators were applied: one scaled by fecal bacterial loss, the other by MAC supply derived from national food surveys, including the 2017–2018 National Health and Nutrition Examination Survey (NHANES). Error propagation used Gaussian statistical formulas based on the standard deviations of biological replicates.
Study results
The ex vivo fermentations revealed remarkable biochemical consistency across taxa despite metabolic diversity. Regardless of medium complexity or pH, each strain converted more than ninety percent of carbohydrate carbon into fermentation acids, validating the measured per-biomass rates as descriptors of anaerobic growth. Averaged across 22 representative species, glucose or maltose uptake clustered tightly, whereas the blend of secreted products differed: Bacteroides favored succinate, Lachnospiraceae produced butyrate, and Enterobacteriaceae generated formate. Weighting these laboratory rates by genus abundance in 219 healthy metagenomes suggested that about 84% of community biomass at the genus level behaves similarly, enabling ecosystem-scale extrapolation.
Applying the excretion coefficients to a 1970s British diet produced convergent point estimates. A fecal-based calculation started with an average 30-gram daily dry-stool output containing approximately 16 grams of bacterial biomass; multiplying by the composite excretion coefficient yielded roughly 470 millimoles of acids per day. A dietary approach traced 36 grams of MACs escaping upper-gut digestion; the carbon needed to regenerate the corresponding 16 grams of biomass predicted a nearly identical 450 millimole daily acid harvest. Less than 2% of this flux left the host in feces, implying near-complete colonic absorption. Protein and mucin fermentation contributed at most one-fifth of the total, confirming carbohydrates as the principal microbial fuel.
Scaling the framework across diets demonstrated that food, not microbial composition, drives energy capture. Processing NHANES dietary records yielded a median harvest of 286 millimoles, well below the British benchmark. By contrast, seasonal diets of Tanzanian Hadza hunter-gatherers, rich in fibrous tubers, generated up to 1,000 millimoles daily. Variation in bacterial community structure altered the relative amounts of acetate, propionate, butyrate, and lactate but changed total production marginally, producing coefficients of variation below ten percent for total acids yet above 30% for individual metabolites.
Translating the fluxes into energy equivalents showed that gut microbes supply between 1.7 and 12.1% of human daily energy expenditure, but more than 21% in laboratory mice because their higher intake of resistant carbohydrates amplifies microbial fermentation. Notably, laboratory mice were fed autoclaved chow, which may further increase the proportion of energy derived from fermentation. Because butyrate supports colonocyte adenosine triphosphate (ATP) production and acetate modulates hepatic gluconeogenesis, lower acid yields from refined diets could contribute to metabolic disease risk, spotlighting dietary fiber as an important focus for public health strategies worldwide in the coming decade.
Conclusions
To summarize, quantitative integration of microbial physiology, diet, and host digestion reveals that colonic bacteria supply a modest but diet-sensitive slice of human energy, returning most MACs to the body as SCFAs. The total flux, about 2–5% of daily expenditure in Western settings, can triple under fiber-rich diets, whereas shifts in community structure mainly reshape the acid mix rather than the sum.
These findings clarify discrepancies between stool concentrations and metabolic impact, expose limitations of mouse extrapolations, and affirm dietary fiber enrichment as a potentially scalable intervention to amplify beneficial microbial energy transfer and improve cardiometabolic risk profiles.
Future studies incorporating dynamic microbiome changes, explicit modeling of cross-feeding, and broader metabolite profiling will be important to further refine these quantitative insights into host-microbiota metabolic interactions.
Journal reference:
- Arnoldini, M., Sharma, R., Moresi, C., Chure, G., Chabbey, J., Slack, E., & Cremer, J. (2025) Quantifying the varying harvest of fermentation products from the human gut microbiota, Cell. DOI: 10.1016/j.cell.2025.07.005 https://www.cell.com/cell/fulltext/S0092-8674(25)00794-9