Alzheimer's disease (AD) is an age-associated neurodegenerative disease. A 2021 report revealed that over 55 million individuals have dementia globally, with low- and middle-income countries accounting for 60% of the estimate. Given the aging population, AD prevalence and related economic burdens are expected to increase. Currently, treatments that can cure AD or modify its pathological progression are lacking.
As such, effective management and regulation of associated factors have been paramount. Diet is implicated in AD prevention and progression, with vitamin A as a potential prophylactic and therapeutic strategy. Non-genomic actions of vitamin A in the brain have crucial roles, and modulating them may improve brain function and offer therapeutic avenues.
 Study: Dietary vitamin A modifies the gut microbiota and intestinal tissue transcriptome, impacting intestinal permeability and the release of inflammatory factors, thereby influencing Aβ pathology. Image Credit: Nefedova Tanya / Shutterstock
Study: Dietary vitamin A modifies the gut microbiota and intestinal tissue transcriptome, impacting intestinal permeability and the release of inflammatory factors, thereby influencing Aβ pathology. Image Credit: Nefedova Tanya / Shutterstock
About the study
In the present study, researchers investigated the effects of dietary vitamin A on the intestinal transcriptome, inflammation, gut microbiota, and amyloid-β (Aβ) pathology. Thirty APP/PS1 mice (AD mouse model) were randomly assigned to three groups based on body weight. Mice received a diet with deficient (VAD), normal (VAN), or enriched (VAS) levels of vitamin A. Body weight and food intake were recorded every week.
Animals were euthanized after 12 weeks. Sera and fecal samples were collected. Tissues from the brain and the gut were harvested. Serum levels of intestinal permeability markers, such as D-lactate and diamine oxidase (DAO), and cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, were measured using enzyme-linked immunosorbent assay (ELISA). Neurobehavioral functions were assessed in a six-day Morris water maze test.
Accordingly, mice were trained for four days, followed by a 60-second search for a hidden platform. A detection test was performed after five days of training, and the time to find the platform was determined. Two trials were administered on the concluding day; one required locating the platform's position in its absence within 60 seconds, and in the other, the time to locate the platform was measured.
Further, morphometric and immunohistochemical analyses were performed to assess Aβ deposition in the brain. 16S rRNA sequencing was performed to analyze the gut microbiota. Transcriptomic analysis of the intestinal tissue was also performed. Serum vitamin A levels were quantified by liquid chromatography-mass spectrometry.
Findings
There were no notable changes in body weight and dietary intake between groups throughout the intervention. However, at distinct time points, the VAS and VAD groups had lower body weight and food intake than the VAN group. The mean vitamin A levels were 382.61 ng/ml in the VAD group, 548.32 ng/ml in the VAN group, and 640.85 ng/ml in the VAS group. Analysis of variance indicated a significant group difference.
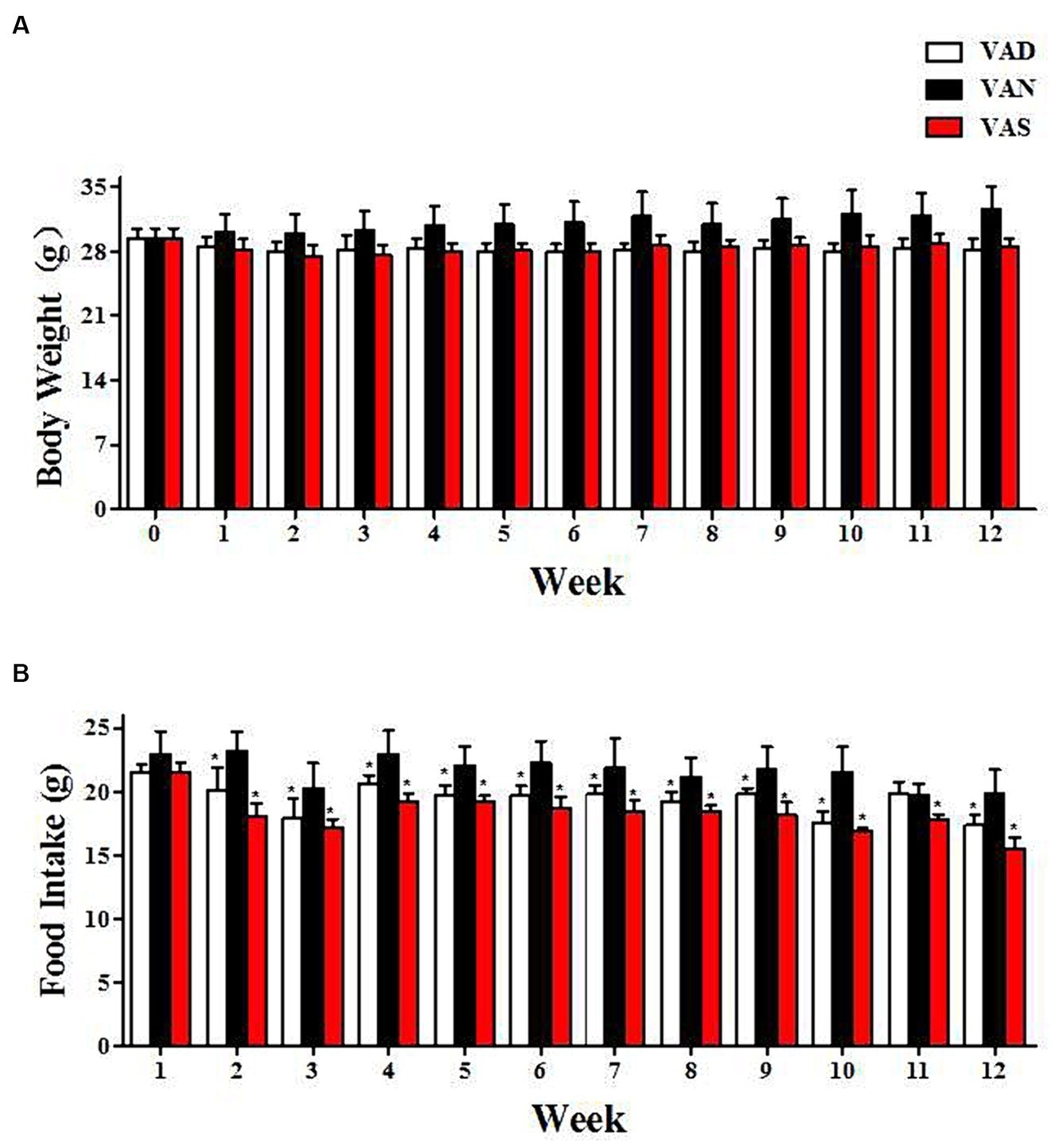
The impact of vitamin A on dietary intake and body weight changes in APP/PS1 mice. (A) The changes in body weight of APP/PS1 mice among different groups. (B) The changes in dietary vitamin A intake among different groups of APP/PS1 mice. *Indicates compared with the VAN group, p < 0.05 indicates statistical significance
Mice in the VAN group could directly locate the platform and move to its position even when removed. These mice took less time to find the platform than other groups. Moreover, the VAS group took less time than the VAD group. There were no significant differences in the time spent finding the platform between VAS and VAN groups. When the platform was removed, the VAN group repeatedly crossed the platform area.
Mice in the VAD group showed significantly greater Aβ deposition in the brain than in other groups. The VAS group also had higher Aβ deposition than the VAN group, albeit not statistically significant. The Shannon index was higher in the VAS and VAN groups than in the VAD group; however, it was similar between the VAS and VAN groups. VAN and VAS groups had greater microbial diversity than the VAD group.
Overall, 571 genes with differential expression were identified between the VAS and VAD groups. Between VAN and VAD groups, 313 differentially expressed genes were identified. Further, there were 243 genes with differential expression between VAN and VAS groups. DAO and D-lactate levels were significantly elevated in the VAD group compared to the VAS and VAN groups. Mice in the VAD group exhibited more significant levels of inflammatory cytokines than other groups.
Conclusions
In sum, the study showed that a 12-week VAD diet reduced serum levels of retinol, impaired cognition, and increased Aβ pathology in mice. On the contrary, a diet enriched with vitamin A increased retinol, reduced Aβ, and preserved cognition. Overall, the findings highlight the significance of vitamin A in AD pathology and behavior.
Journal reference:
- Wang ZL, Pang SJ, Zhang KW, Li PY, Li PG, Yang C. Dietary vitamin A modifies the gut microbiota and intestinal tissue transcriptome, impacting intestinal permeability and the release of inflammatory factors, thereby influencing Aβ pathology. Front Nutr, 2024, DOI: 10.3389/fnut.2024.1367086, https://www.frontiersin.org/articles/10.3389/fnut.2024.1367086/full