A next-generation nanobody-liposome treatment slashes tumor growth by homing in on a key cancer marker, opening new doors for safer and more effective lung cancer therapy.
 Study: Targeting CD155 in lung adenocarcinoma: A5 nanobody-based therapeutics for precision treatment and enhanced drug delivery. Image Credit: luminance studio / Shutterstock
Study: Targeting CD155 in lung adenocarcinoma: A5 nanobody-based therapeutics for precision treatment and enhanced drug delivery. Image Credit: luminance studio / Shutterstock
In a recent study published in the journal Signal Transduction and Targeted Therapy, researchers in South Korea developed nanobody (Nb)-conjugated liposomes targeting cluster of differentiation 155 (CD155) for treatment in lung adenocarcinoma (LUAD).
Lung cancer is the leading cause of global cancer-related mortality. While targeted treatments have transformed the therapeutic landscape of non-small cell lung cancer, various clinical limitations impede long-term outcomes. The systemic toxicity of small-molecule chemotherapies and the poor tumor penetration of biologics further complicate disease management, highlighting the need for novel treatments with improved delivery efficiency and high specificity.
Of late, CD155 has emerged as a promising therapeutic target. Its overexpression in various malignancies, including LUAD, has been linked to poor prognosis. Nbs are single-domain, antigen-binding fragments that offer unique benefits in drug delivery, including thermal stability, solubility, tissue penetration, and large-scale production. These properties allow for tumor-targeted delivery and integration into liposomal and nanoparticle platforms.
The study and findings
In the present study, researchers developed anti-CD155 Nb-conjugated liposomes as a targeted drug delivery system for LUAD. From an Nb library of over 1011 unique variants, they identified 50 clones that specifically bind to CD155. After further screening, the A5 clone was selected for its optimal kinetic profile. Structural analysis revealed that A5 binds with high affinity (Kd = 1.17 nM), forming key hydrogen bonds (Ser102-Asp130, Glu114-His103, Asp99-Thr39) and hydrophobic contacts that ensure a stable and specific interaction with CD155.
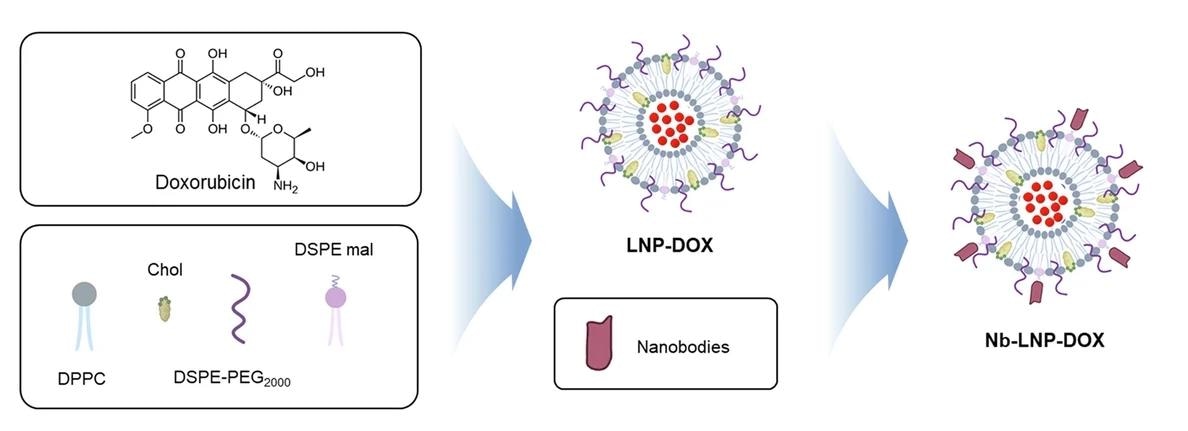
Flowchart of the synthesis of A5-LNP-DOX.
The A5 nanobody demonstrated remarkable performance in lung cancer cell lines with high CD155 levels. It showed binding 20-30 times higher than in normal cells and achieved over 80% cellular uptake, significantly exceeding the 25% uptake rate of a conventional CD155 antibody. This superior efficiency meant that A5 was effective at concentrations 10-fold lower than those required for the conventional antibody.
Functionally, A5 treatment led to a dramatic, over 50% decrease in cancer cell migration and invasion in CD155-high cells, an effect not seen in CD155-low cell lines. The study revealed that this was achieved by disrupting the cell's focal adhesion signaling. The investigation into its mechanism revealed the downregulation of paxillin (PXN), a key focal adhesion scaffold protein, which resulted in cell shrinkage and a 4-5 fold reduction in PXN localization at the cell membrane. Importantly, this effect was highly specific and did not alter other known cancer pathways like EMT or AKT/ERK.
To confirm the clinical relevance of this finding, the team performed immunohistochemical staining on tumor specimens from lung cancer patients. They found that PXN and CD155 had significantly higher expression in tumor samples than in normal tissues and showed a strong correlation (R=0.554, p<0.0001). Crucially, patients with high levels of both CD155 and PXN had significantly worse survival outcomes (p=0.0018), establishing the CD155-PXN axis as a key prognostic marker.
Building on these findings, the researchers developed ~55 nm liposomes loaded with the anticancer drug, doxorubicin (DOX), and conjugated them with A5 Nbs (A5-LNP-DOX). This targeted delivery system showed a 2-3 fold higher uptake in cancer cells than untargeted liposomes and doubled the cytotoxic effect, leading to a significant increase in cancer cell death and apoptosis markers.
The antitumor activity of A5-LNP-DOX was then assessed in advanced animal models. In an orthotopic lung cancer model, A5-LNP-DOX exhibited the highest tumor suppression, reducing the lung tumor burden to just 4%, compared to 40% in the untreated control group. The treatment also demonstrated robust antitumor activity in a human lung cancer organoid (LCO) xenograft model. Systemic toxicity studies showed that the treatment was well-tolerated, with no signs of major organ damage.
Conclusions and future directions
In sum, the study developed the A5 Nb and showed its ability to modulate tumor phenotypes with superior potency and cellular uptake versus conventional antibodies. It inhibited the migration and invasion of CD155-high lung cancer cells via specific disruption of the newly identified CD155-PXN-focal adhesion axis. The A5-LNP-DOX drug delivery platform demonstrated robust and targeted antitumor activity across multiple advanced in vivo models.
The authors also highlighted key limitations that guide future work. First, the LCO xenograft model used only a single patient-derived line, so further validation is needed across diverse patient samples. Second, because the A5 nanobody does not bind to mouse CD155, a full assessment of on-target, off-tumor toxicity will require studies in humanized transgenic models. Finally, while soluble CD155 isoforms were negligible in the tested models, their potential role as therapeutic decoys warrants consideration in future clinical scenarios.
Journal reference:
- Noh K, Yi S, Kim H, et al. (2025). Targeting CD155 in lung adenocarcinoma: A5 nanobody-based therapeutics for precision treatment and enhanced drug delivery. Signal Transduction and Targeted Therapy, 10(1). DOI: 10.1038/s41392-025-02301-z, https://www.nature.com/articles/s41392-025-02301-z