As scientists uncover how age-altered antibodies ignite inflammation and tissue decline, a new era of immune-based anti-aging therapies is beginning to emerge.
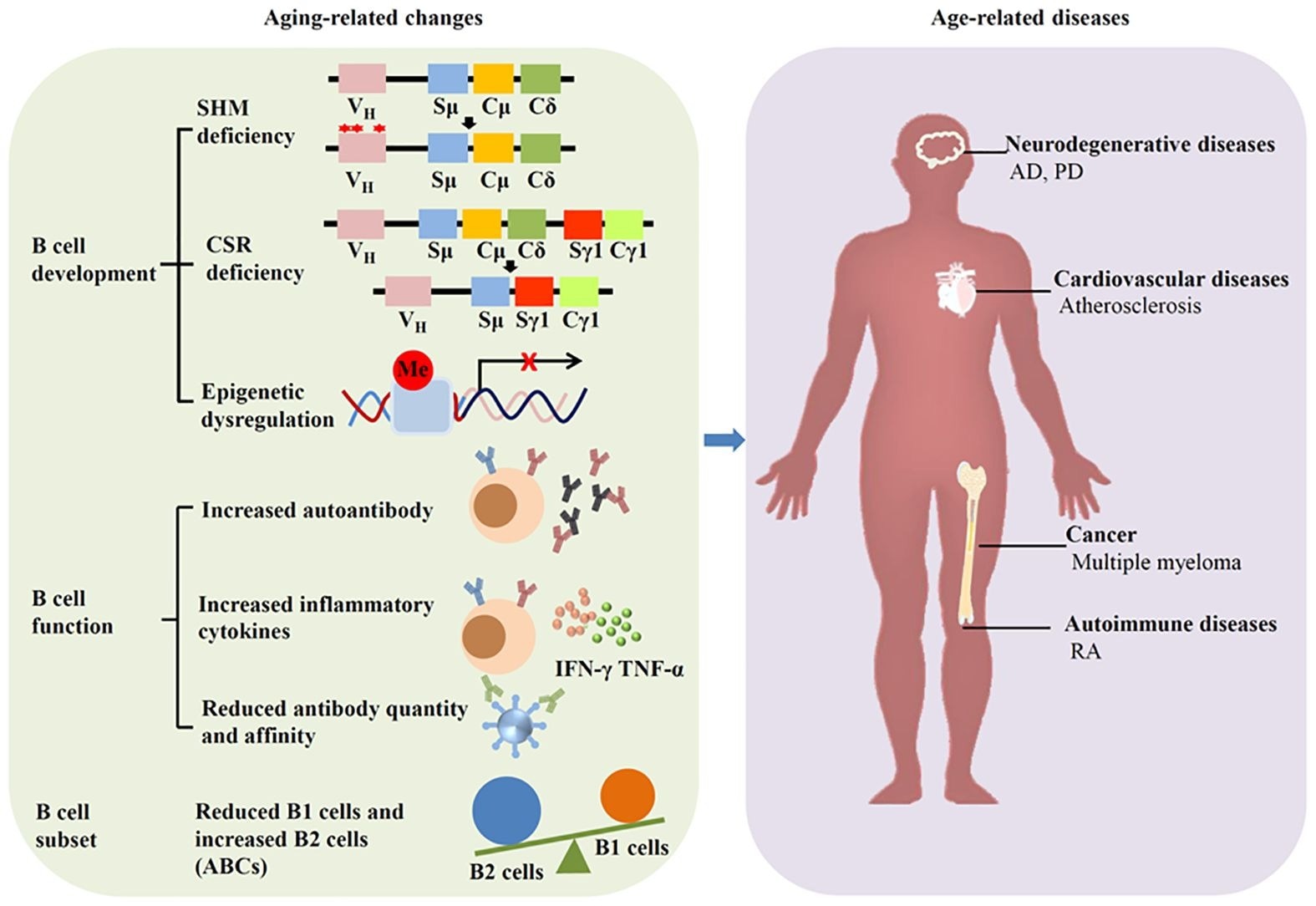
Aging affects B cells and induces diseases. Aging can alter the development, function and subset of B cells, leading to age-related diseases such as neurodegenerative diseases, cardiovascular diseases, autoimmune diseases, and cancer.
In a recent review published in the journal Frontiers in Immunology, researchers summarized literature on immunoglobulin changes linked to aging and related diseases.
Aging is one of the leading causes of chronic diseases, such as cancer, neurodegenerative diseases, and cardiovascular diseases, which eventually will lead to death. Among the various factors contributing to aging, cellular senescence is regarded as a hallmark and driver of tissue dysfunction. Senescent cells accumulate in organs and tissues during aging, and their clearance can mitigate aging and related diseases. Studies have reported immunoglobulin accumulation adjacent to senescent cells.
Reports also indicate increased expression of immunoglobulin-related genes in aged tissues, suggesting that IgG accumulation has been proposed as a hallmark of human aging, partly due to its ability to trigger cellular senescence and chronic inflammation. Age-related changes in IgG glycosylation, such as reduced galactosylation and sialylation, further enhance pro-inflammatory signaling and contribute to inflammaging through the REST/NF-κB pathway. Gaining insights into the mechanisms underlying aging can improve the quality of life and lifespan. In the present study, researchers reviewed the literature on immunoglobulin changes associated with aging and related diseases.
Aging and B cells
Aging leads to substantial changes in the immune system, primarily in the humoral compartment mediated by B cells. This phenomenon, immunosenescence, can elevate the severity and frequency of infectious diseases and decrease responses to vaccination. Human and murine bone marrow show diminished B cell precursors during aging, attributable to microenvironmental changes associated with age.
Moreover, B1 cells, which are fetal liver-derived, decrease with aging in older individuals, along with a reduction in the function and number of natural antibodies. B2 cells (conventional B cells) exhibit functional impairments associated with aging, which manifest as defective isotype switching in aged cells. Although peripheral B2 cells remain unchanged in aged mice, follicular and marginal zone B2 cells decrease with age, accompanied by an increase in autoantibodies.
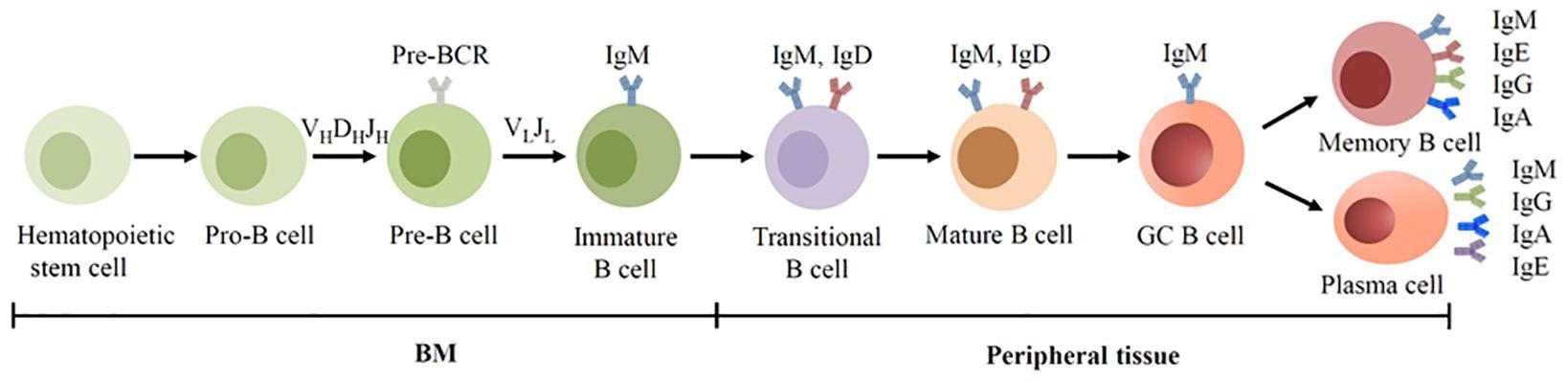
BCR expression during human B cell development. B cells are derived from hematopoietic stem cells in the BM, and acquire BCRs through V(D)J recombination of Ig genes in the early development. In the GC, B cells undergo SHM and CSR, allowing the BCR switched from IgM to other isotypes.
Aging and immunoglobulins
Aging can elevate immunoglobulin M (IgM) levels in mice, and aged mice express more serum IgM than their younger counterparts. However, IgM levels decline with age in humans, and overall serum immunoglobulins may remain stable or mildly increase, with shifts toward higher IgG and IgA and lower IgM and IgD. For instance, lower levels of atheroprotective IgM (such as anti-phosphorylcholine) have been linked to vascular aging and atherosclerosis, including in systemic lupus erythematosus (SLE) cohorts. Conversely, elevated urinary IgM has been associated with vascular injury and endothelial dysfunction, highlighting the dual role of IgM in aging.
Research suggests that IgG accumulation is a hallmark of aging. Besides, IgG accumulation can lead to cell senescence and contribute to tissue aging. Further, recent studies indicate that band 3 protein, predominantly expressed on erythrocytes, is crucial for erythrocyte senescence signaling. The Band 3 anion exchanger produces a senescent cell antigen that binds to serum IgG, which is crucial for erythrocyte removal. Notably, senescent erythrocytes bind to more IgG than young erythrocytes. Band 3 protein shows higher degradation in neurodegenerative diseases, including AD and PD, producing more senescent cell antigen.
IgG also accumulates in white adipose tissue and induces adipose tissue degeneration in aging. The neonatal Fc receptor (FcRn), which prevents IgG degradation, contributes to this accumulation, and FcRn inhibition has been shown to reduce tissue IgG burden. Further, IgA exhibits significant changes with aging; specifically, serum IgA levels increase. C-C motif chemokine ligand 25 (CCL25) is a chemokine that recruits IgA-secreting cells into the intestinal lamina propria. Aging can decrease CCL25 expression, affecting gut immunity. Moreover, gut microbiota dysbiosis has been detected in IgA-deficient humans. Emerging evidence suggests IgA-microbiome interactions may influence mucosal senescence and systemic immune homeostasis.
Therapeutic potential of immunoglobulins in aging
Immunoglobulin therapy exerts immunomodulatory and anti-inflammatory effects and has been used in immunodeficient diseases and other immunological conditions. However, its therapeutic efficacy in aging is yet to be clarified. A recent study showed that IgG suppression can effectively reduce aging in mice. Meanwhile, other studies have found that an antisense oligonucleotide against neonatal Fc receptor (FcRn) can inhibit IgG accumulation in adipose tissue. IgM transfer has also been explored for the induction of immune tolerance and the reduction of autoimmunity, indicating potential anti-aging applications.
Inflammaging is the hallmark of aging and immunosenescence; anti-inflammatory treatments are regarded as potential anti-aging therapies. Interventions targeting B cells could mitigate aging by regulating senescence, inflammation, and immunoglobulin production. For instance, rituximab is a monospecific antibody targeting B cells; it can alleviate multiple sclerosis, atherosclerosis, and rheumatoid arthritis. However, monospecific antibodies have limited efficacy as they only target a specific molecule. As such, bispecific antibodies, antibody-drug conjugates, and emerging platforms such as chimeric antigen receptor T (CAR-T) cell therapy have been introduced to augment therapeutic efficacy.
Concluding remarks
Overall, aging is a highly interlinked process in which immune responses decline gradually. Growing evidence suggests that aging regulates immunoglobulin function and production through epigenetic modification, immunosenescence, microbial disturbances, and inflammation. Beyond B cells, immunoglobulin is produced by other cells, including macrophages, but the function of macrophage-derived immunoglobulin remains unclear. Further studies into non-B-cell-derived immunoglobulins and IgG glycosylation dynamics may reveal novel biomarkers and therapeutic targets in aging.