Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), primarily infects cells in the respiratory tract. Emerging in China in December 2019, the virus soon spread across the globe, progressing into a pandemic that presents a significant threat to global public health.
As of today, it has caused more than 42.9 million confirmed cases and over 1.15 million deaths across the world. The USA, India, and Brazil are the worst-hit countries, with over 50% of the total cases. Since there are no drugs or vaccines for COVID-19 yet, current treatment strategies mainly focus on supportive care, symptom management, and isolation of infected people.
Current evidence shows that SARS-CoV-2 spreads mainly through human-to-human transmission via respiratory droplets. The standard diagnostic testing method used to detect SARS-CoV-2 is the real-time reverse transcription-polymerase chain reaction (RT-PCR) test. Antibody tests such as enzyme-linked immunosorbent assay (ELIZA) are used at later stages to look for IgM or IgG antibodies produced against SARS-CoV-2. Although COVID-19 symptoms range from none to mild and severe pneumonia, surveillance is only possible based on the confirmed cases. This could lead to underestimating the total number of positive cases because people with mild and asymptomatic infections may not get tested.
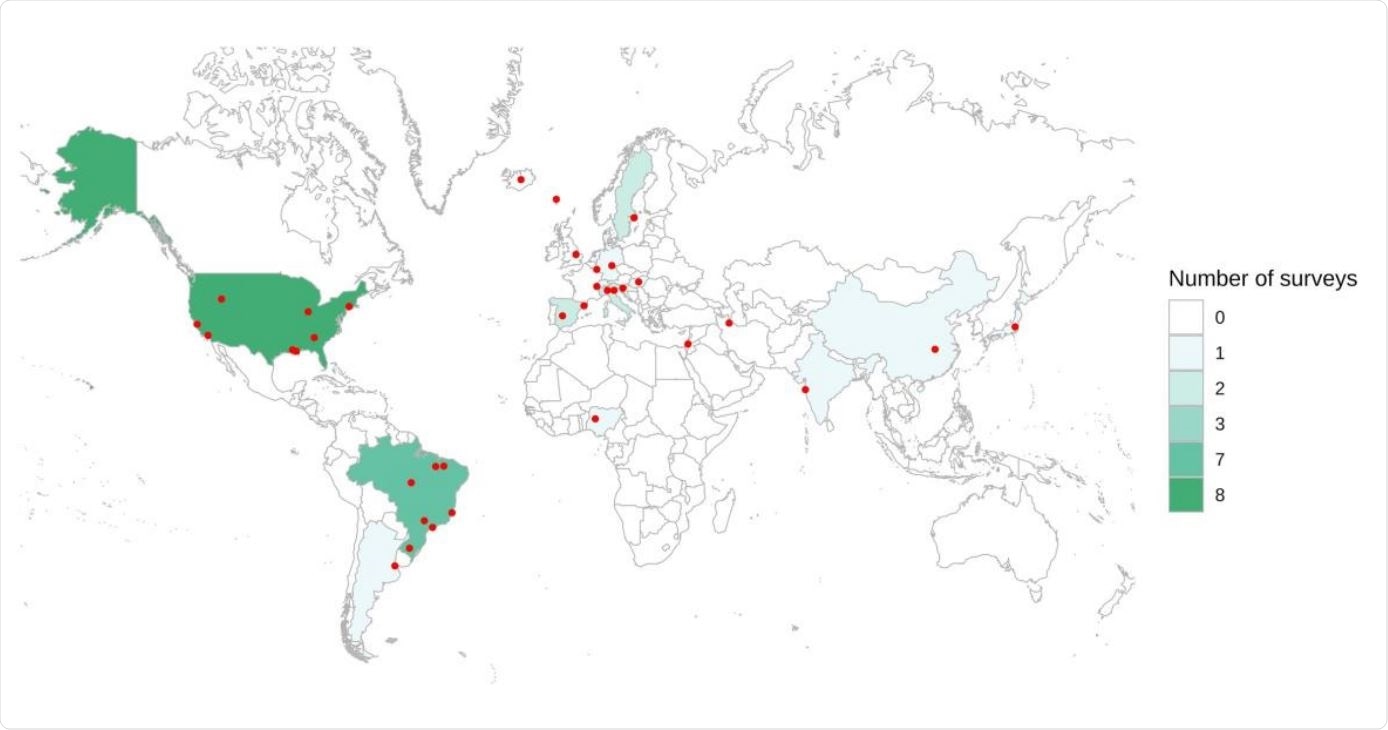
Map of countries and specific regions with prevalence surveys. Red dots represent regions and cities where the initiatives were performed. In nationwide studies, the point was placed in the center of the country.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Qualitative assessment of population-based COVID-19 prevalence surveys in 19 countries
Population-based surveys on the prevalence of COVID-19 help establish the epidemiology of infection and the role of people with asymptomatic and mild infections in disease transmission. These surveys also allow more accurate decision-making regarding reopening policies when approved pharmacological interventions are not available.
A team of researchers from the Federal University of Health Sciences of Porto Alegre and the Federal University of Rio Grande do Sul performed a systematic review to assess these population-based COVID-19 prevalence surveys' reliability and biases qualitatively. Their study is published on the preprint server medRxiv*.
The team assessed each survey's quality using the Joanna Briggs Institute Critical Appraisal Checklist for Prevalence Studies, which evaluates 9 domains. They are sample and frame adequacy, recruitment method, sample size, study subjects and setting, coverage, diagnostic methods, the reliability and standardization of measurements, statistical analysis, and the response rate.
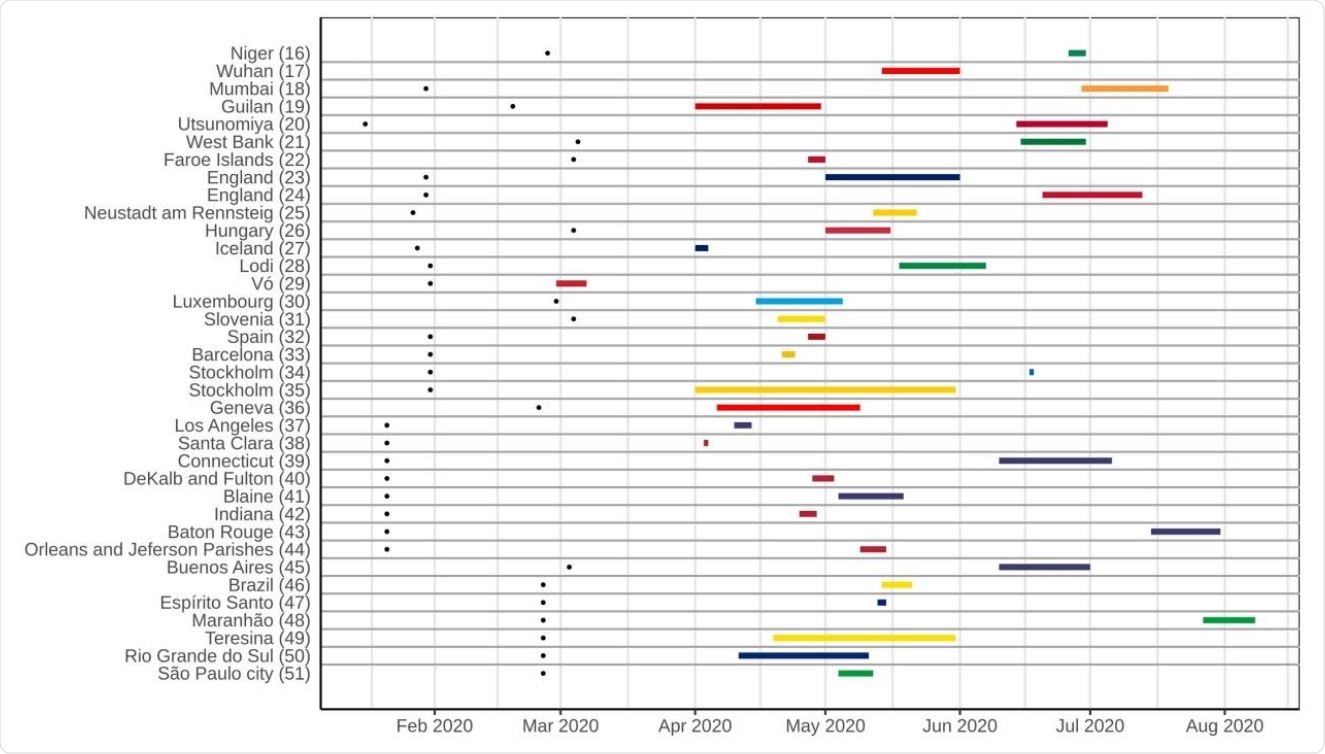
Timeline of population-based COVID-19 prevalence surveys conducted worldwide, with the duration of each survey and an overview of the most represented periods. Black dots on the left represent the date of the first confirmed case in the country of each survey.
Study finds intermediate risk of bias in American and European studies and low risk of bias in Asian studies
The researchers examined data related to 37 surveys from 19 countries across Europe and America that used antibody testing. They found that the sample sizes and prevalence estimates were highly heterogeneous and also observed disproportional prevalence in minority communities. The team detected a significant risk of bias in 4 domains: sample size, data analysis with sufficient coverage, measurements in standard way, and response rate. They found few consistent patterns for high risk of bias through correspondence analysis. They also found an intermediate risk of bias in American and European studies (prevalence >1%) and low risk of bias in Asian studies (prevalence <1%).
“In this analysis, few consistent patterns were observed for studies with a high risk of bias, indicating that particular methodological choices of each study may affect its quality, not choices that are being made in many studies worldwide.”
Low sample size and low response rate influenced the reliability of prevalence estimates
Important limitations of the researchers' surveys were low sample size and low response rate, both of which significantly influence the reliability of prevalence estimates. As repeated cross-sectional studies had very distinct prevalence estimates due to the ascending infection curve, different sample sizes were needed for each period. Unfortunately, some of the studies analyzed did not have adequate sample sizes in all rounds.
According to the authors, the high numbers (n=53; 15.9%) of "unclear" response reported in these surveys may be due to accelerated publication and the lack of checklists' knowledge. The team recommends the use of standardized checklists for planning, execution, and reporting of prevalence studies.
“Although the number of studies were low and the correspondence analysis presents some outliers due to the low representativeness of some categories, these conclusions were highly consistent and showed some important aspects of population-based COVID-19 prevalence studies associated with the methodological quality.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Population-based prevalence surveys during the COVID-19 pandemic: a systematic review Vinícius Bonetti Franceschi, Andressa Schneiders Santos, Andressa Barreto Glaeser, Janini Cristina Paiz, Gabriel Dickin Caldana, Carem Luana Machado Lessa, Amanda de Menezes Mayer, Julia Gonçalves Küchle, Paulo Ricardo Gazzola Zen, Alvaro Vigo, Ana Trindade Winck, Liane Nanci Rotta, Claudia Elizabeth Thompson medRxiv 2020.10.20.20216259; doi: https://doi.org/10.1101/2020.10.20.20216259, https://www.medrxiv.org/content/10.1101/2020.10.20.20216259v1
- Peer reviewed and published scientific report.
Franceschi, Vinícius Bonetti, Andressa Schneiders Santos, Andressa Barreto Glaeser, Janini Cristina Paiz, Gabriel Dickin Caldana, Carem Luana Machado Lessa, Amanda de Menezes Mayer, et al. 2021. “Population-Based Prevalence Surveys during the Covid-19 Pandemic: A Systematic Review.” Reviews in Medical Virology 31 (4): e2200. https://doi.org/10.1002/rmv.2200. https://onlinelibrary.wiley.com/doi/10.1002/rmv.2200.