The current pandemic of COVID-19 and the race to discover effective drugs and vaccines to counter the deadly spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have made it imperative to understand how the virus works in the body to cause disease and death.
At present, most fatalities are thought to be the result of ARDS (acute respiratory distress syndrome), based on post-mortem analysis. However, a new Italian study published on the preprint server medRxiv* in August 2020 shows that this may not be an adequate explanation, since ARDS is a nonspecific diagnosis. Instead, they say, antemortem lung biopsy shows a “Covid pattern” of acute lung injury, which could guide the application of therapeutics.
Ventilation-Associated Lung Injury and Vascular Factors
Prolonged periods of mechanical ventilation causes many factors to act on the lung and airways, including ventilator-associated pneumonia, oxidative damage due to high-pressure oxygen, bacterial super-infections, and tissue breakdown due to autolysis. This must be taken into account when interpreting the post-mortem findings.
Many investigators have found the presence of thromboembolism in these specimens, which suggests that endothelial injury plays a part in producing these lesions. This is supported by finding small vessel disease, T cell infiltration around the blood vessels, and endothelial lesions. Such a constellation of thromboembolism, excessive coagulation activity, and vascular alterations, is common to ARDS due to any cause.
Lung Biopsy to Uncover Pathogenesis
The current study focused on antemortem lung biopsy as a means of providing a better understanding of the pathogenesis of lung injury in COVID-19. In addition, these biopsies would reveal the presence of super-infections, and non-infectious complications, in patients on mechanical ventilation.
The current prospective observational study included the histological and immunohistochemical examination of lung biopsy specimens. There were 23 patients, of which 12 and 11 had a biopsy performed in the early (within 15 days of symptom onset) and late (after 15 days of symptom onset) phase of COVID-19.
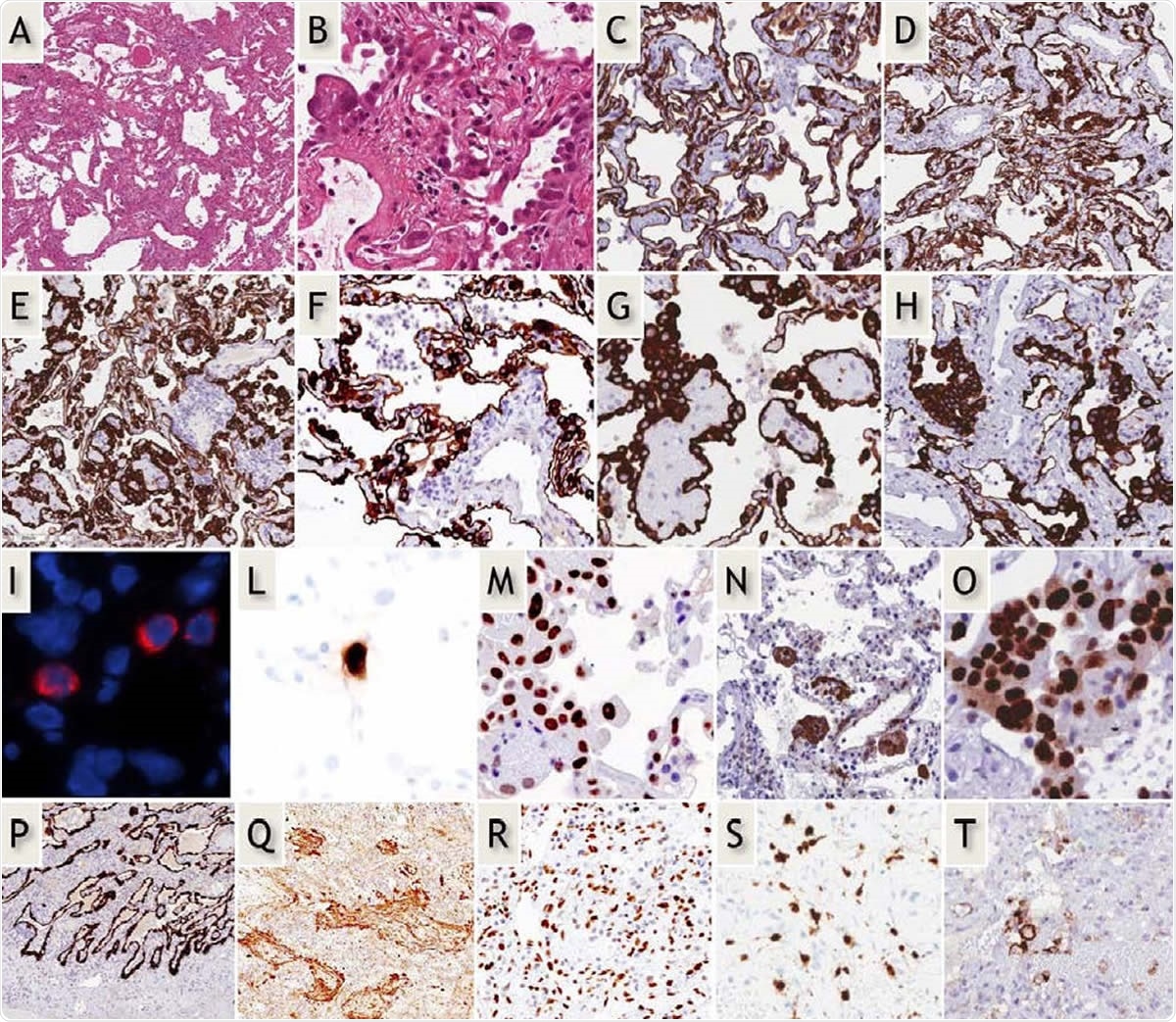
Early-phase COVID-19 pneumonia. H&E (A,B): Parenchymal structure is variably altered by AECII hyperplasia, vascular enlargement and interstitial thickening. CK7 (G-H): AECII form variable small nodules, aggregates and pseudo-papillary sprouts. Grade-1 (C,D) and -2 (E-H) Covid-19 histological patterns were defined by the extent of AEC II hyperplasia. In situ demonstration of AECII infected by SARS-CoV-2 (I): cytoplasmic (red) signals are evidenced in scattered cells recognized as AECII by morphology and location. In situ analysis of IL-6 mRNA expression (L): strong signal is evidenced in scattered AECII. Ph-STAT3 immunohistochemistry (M): strong signal demonstrated in most AECII. TBB3 immunohistochemistry (N): strong signal in AECII. Interstitial dilated spaces are negative. Ki67 immunohistochemistry (O): elevated (>50%) proliferation in AECII. Late-phase Covid-19 pneumonia. CK7 (P): typical DAD-presentation with homogeneous “lepidic” alveolar covering by AECII. TBB3 (Q) strong reaction in myofibroblast-rich areas. phSTAT3 (R): diffuse nuclear expression in AECII, macrophages and stromal cells. IL-6 mRNA in-situ (S): increased numbers of positive cells. PD-L1 (T): negative results in most blood vessels.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Early Disease – the COVID Pattern
The researchers found that early phase biopsies had patchy acute lung injury (ALI), hyperplasia of alveolar epithelial type II cells (AECII), and vascular abnormalities (disorderly formation of blood vessels, thickening of alveolar capillaries leading to a colander-like pattern, dilated, tortuous and thickened pulmonary venules, and CD4 T cell infiltration. There were no signs of vasculitis or endothelial inflammation. However, the hyaline membranes characteristic of diffuse alveolar damage (DAD), which is typical of ARDS, were absent. This pattern was called the “COVID pattern.”
Late Disease Pattern
Late phase biopsies showed disrupted alveolar structure, with the ALI showing signs of organization. Congestion of the vascular architecture was prominent. There were 7 out of 11 biopsies showing CK7+ epithelial cells scattered within a highly cellular tissue comprising interstitial myofibroblasts, inflammatory cells, and blood vessels. In only one case was a hyaline membrane present.
Proliferation Resulting from Alveolar Infection
Studies using in situ hybridization showed that the alveolar cells were infected, as expected, since these cells express ACE2. However, infected cells were fewer in more affected areas where the AECII appeared to be actively proliferating, as marked by the presence of the marker Ki67. On the other hand, their proportion was lower in relatively normal areas of lung parenchyma. Along with the lack of markers indicating apoptosis, the researchers argue that this means that “AECII do not die following infection but rather receive proliferative signals.” They noted the unusual presence of nodular clumps of activated AECII in COVID-19.
Non-DAD Pattern
The significant differences between this picture and DAD include the absence of hyaline membranes, which are formed from the lysis of AECII. This indicates that the hypoxia seen in late COVID-19 is not related to alveolar loss. In fact, extensive patches of normal alveoli are prominent in early disease rather than widespread interstitial fibrosis expected in DAD.
However, late-phase biopsies showed the presence of interstitial fibrosis, as seen in post-mortem COVID-19 studies. The researchers say this could be attributed to the use of high positive end-expiratory pressure (PEEP) in these critically ill patients, causing alveolar damage.
Cell Markers of Immune Tolerance
In the early phase, lung cells and endothelial cells showed striking features such as elevated expression of phosphor-STAT3 (pSTAT3), PD-L1, and IDO-1, unlike control samples. The latter molecules are inhibitors of inflammatory immune pathways. The high pSTAT3 levels in the nucleus of both AECII and endothelial cells, as well as of IL-6 in the AECII alone, could indicate that the NF-kB/STAT3 pathway is being activated not by the innate immune antiviral response mediated via IL-6, but by pro-inflammatory factors secreted by the lung parenchyma. PD-L1 is also elevated by the signaling pathway involving IFN-γ, JAK, and STAT. Typically, lung tissue expresses low levels of IDO-1, except post-viral infection, when it is probably induced byIFN-γ secreted by activated lymphocytes.
In the later phase, both of these markers are secreted by a range of cells, including lung stroma and macrophages, as well as AECII. These molecules exercise negative feedback on the immune system, and induce immune tolerance of tumor cells.
Thus, both these endothelium-secreted molecules are part of the lung’s response to injury, suggesting that in COVID-19, they magnify the immune tolerance caused by innate immune suppression within the lung. Not only so, IDO-1 also modulates vascular tone and protects against pulmonary hypertension. Its overexpression in COVID-19 could be the cause of the reduction in pulmonary vascular tone and the dilated tortuous pulmonary vessels in early COVID-19 pneumonia. This could also be the reason for the hypoxia that is seen in these patients even before pneumonia becomes severe because it causes an increase in alveolar dead space.
Vascular Changes
Abnormal blood vessels are also a part of COVID-19 lung changes from the beginning and continue to be observed until the end. Half of the early-phase patients showed high lymphocyte counts on bronchoalveolar lavage (BAL), mirroring the presence of perivascular lymphocytes. This redistribution of lymphocytes to the lung tissue could be part of the reason for the low lymphocyte counts in peripheral blood seen in COVID-19 patients, especially CD4 cells. On the other hand, cytokine-induced apoptosis and T cell exhaustion are more likely to account for low CD8 T cells counts.
In late disease, D-dimer levels were high, linked to capillary fibrin deposition. Alveolar macrophages expressed some atypical molecular markers such as DC-Lamp/CD208, CD206, CD123/IL3AR. These resemble the monocyte changes seen when exposed to the lung epithelium, which induces the release of inflammatory cytokines precipitating unregulated cytokine release.
Implications
The researchers point out that their findings indicate a “complex scenario where severe derangement of the cross-talk between innate and adaptive immune mechanisms are triggered by viral infection.” Both inflammatory and tolerance-promoting factors are produced in the lung by different cell types. An imbalance between these states may determine whether the patient progresses to severe disease or recovers.
The pattern of pneumonia seen in many early COVID-19 infections is not the typical DAD found on post-mortem examination of these cases. The prominent ‘Covid pattern’ was found in the early phase of the disease, comprising abnormal epithelial and endothelial cell phenotypes. The reversibility of these findings marks them out from those of classical DAD. Again, the vascular changes are clearly different from those seen with ARDS, and this may help to develop a theory of disease in COVID-19. The researchers observe that, as with tumor immunity, “These observations may have major therapeutic implications, justifying studies of early interventions aimed at mitigating inflammatory organ injury.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Ravaglia, C. et al. (2020). Acute Lung Injury Evolution in Covid-19. medRxiv preprint. doi: https://www.medrxiv.org/content/10.1101/2020.08.09.20170910v1
- Peer reviewed and published scientific report.
Ravaglia, Claudia, Claudio Doglioni, Marco Chilosi, Sara Piciucchi, Alessandra Dubini, Giulio Rossi, Federica Pedica, et al. 2022. “Clinical, Radiological, and Pathological Findings in Patients with Persistent Lung Disease Following SARS-CoV-2 Infection.” European Respiratory Journal, March, 2102411. https://doi.org/10.1183/13993003.02411-2021. https://erj.ersjournals.com/content/early/2022/02/24/13993003.02411-2021.