Sponsored Content by Bio-TechneReviewed by Louis CastelJan 11 2024
Viral vectors, such as adeno-associated viruses (AAV) and lentiviral viruses (LVV), are recently at the forefront of biotherapeutic development for the support of cell and gene therapies.
As with conventional biologics, such as monoclonal antibodies, the careful manufacture and testing of viral vectors is required to ensure product safety, quality, efficacy, and consistency.
A series of critical qualities and attributes (referred to as CQAs) are assigned to maintain these standards. The physiochemical properties of these vectors determine the identity, purity, stability, and potency of viral vectors.
A major challenge facing the industry today is the need for multiple analytical tools in testing, necessitating significant capital investment, space, and training. This means that more valuable materials are required for characterization, and therefore fewer are available for release.
Capillary electrophoresis (CE) is a powerful technique for characterizing and monitoring several CQAs. Among CE instruments, the Maurice™ CE system stands out by offering comprehensive characterization of viral capsids. This article highlights how Maurice facilitates both CE-SDS and icIEF analysis for AAVs and LVVs.
Materials
Instrument
MauriceFlexTM (ProteinSimple), cIEF, Turbo CE-SDS, and Flex cartridges (as shown in Figure 1).
Figure 1. Maurice CE instrument and cartridges. Image Credit: Bio-Techne
Samples
AAV9 particles (Virovek) at 2 X 1013 VP/mL, LVV particles (Takara) at 1.1 X 1010 TU/mL.
Additional materials
Amicon™ Ultra-0.5 Centrifugal Filter Units, 10K MWCO (Milipore UFC500396), and method-specific reagents (listed in Table 1).
Table 1. Additional reagents used in this study. Source: Bio-Techne
| Reagent |
Vendor |
Part Number |
| Urea powder |
SIGMA |
33247 |
| Triton X-100 reduced |
X100RS |
| CHAPS hydrate |
C9426 |
| Dithiothreitol (DTT) |
D0632 |
| Formamide (>99.5 %) |
47671 |
| Dimethyl sulfoxide (DMSO) |
D2650 |
| 2-Mercaptoethanol |
M6250 |
| ASB -14 |
A1346 |
| Pharmalyte 3-10 |
Cytiva |
17-0456-01 |
| Pharmalyte 5-8 |
17-0453-01 |
| pI marker 5.85 |
ProteinSimple |
102225 |
| pI marker 8.40 |
102229 |
| pI marker 4.65 |
102223 |
| pI marker 9.50 |
101996 |
| 1 % methycellulose |
101876 |
| Maurice CE-SDS PLUS Application Kit |
PS-MAK03-S |
| Maurice Turbo CE-SDS Application kit |
PS-MAK01-TS |
Methods
Imaged cIEF analysis of AAV capsid proteins
1 µL of 160 mM DTT and 7 µL DMSO were added to 5 µL AAV (2 x 1013 GC/mL) and mixed. The mixture was subsequently heated at 70 °C for 10 minutes to denature the sample. After denaturation, the sample was cooled to room temperature, which was maintained until analysis.
The final IEF sample solution comprises 50 % formamide, 2 % Pharmalyte 3-10, 2 % Pharmalyte 5-8, 0.35 % methylcellulose, and pI markers 5.85 and 8.40. The denatured AAVs were focused at 1.5 kV for 1 minute, followed by 3 kV for 12 minutes, and were then imaged with native fluorescence (20–80 second exposures).
Imaged cIEF analysis of LVV capsid proteins
LVV was inactivated in 0.5 % Triton X-100 reduced at room temperature for 20 minutes. The inactivated LVV was denatured for 10 minutes at 95 °C in 16 mM DTT, 1 % CHAPS, and 0.5 % ASB-14. After denaturation, the LVV was diluted 15 times using cIEF master mix to create the final sample solution.
This final solution comprised 9 M urea, 10 % CHAPS, 4 mM DTT, 0.25 % Triton X-100 reduced, 0.01 % ASB-14, 0.35 % methylcellulose, 4 % Pharmalyte 3-10, and pI markers 4.65 and 9.50. The focusing time was 1.5 kV for 1 minute, followed by 3 kV for 8 minutes. Imaging of the samples was conducted with native fluorescence (10–80 second exposures).
Turbo CE-SDS analysis of AAV capsid proteins
Cold acetone (4x the sample volume) was added to 20 μL of the AAV sample and briefly vortexed. The sample was kept at -20 °C for an hour, followed by centrifugation at room temperature for 10 minutes at 15,000 xg to pellet the proteins.
The supernatant was then removed carefully, and the precipitate was allowed to dry for 5 minutes. It was dissolved in 20 μL of CE-SDS PLUS buffer and vortexed. For denaturation, 0.7 M β-mercaptoethanol (β-ME) was added to the buffer, and the solution was incubated for 10 minutes at 70 °C.
The sample was then cooled on ice for 5 minutes and spun with a micro-centrifuge. Distilled water was subsequently added to reach a final volume of 100 μL for Turbo CE-SDS. For analysis with Turbo CE-SDS, samples were injected for 8 seconds at 3500 V and separated for 8 minutes at 4200 V. The entire dataset was analyzed using Compass for iCE software.
CE-SDS PLUS analysis of LVV capsid proteins
LVV particles (1.1 x 1010 TU/mL) were heat-inactivated at 95 °C for 2 minutes. Following inactivation, samples were kept on ice for immediate use or at -80 °C for future use. For protein extraction, cold acetone precipitation was utilized (described above); however, the pellet was not dissolved in 2 % SDS containing 200 mM bicine (pH 5.5).
Results
Characterization of lentiviral capsid proteins
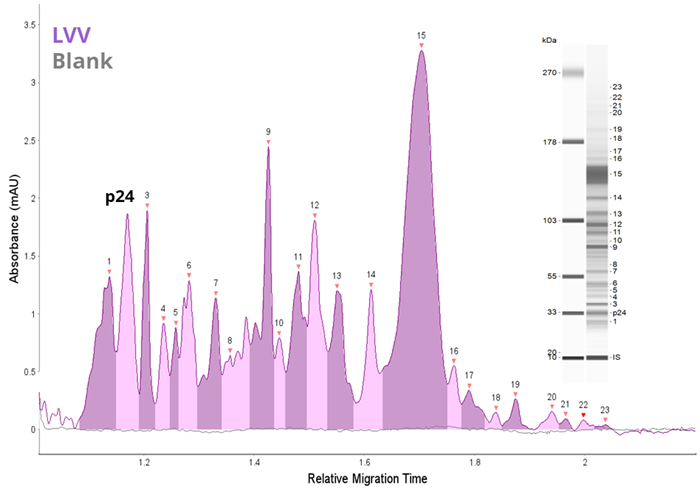
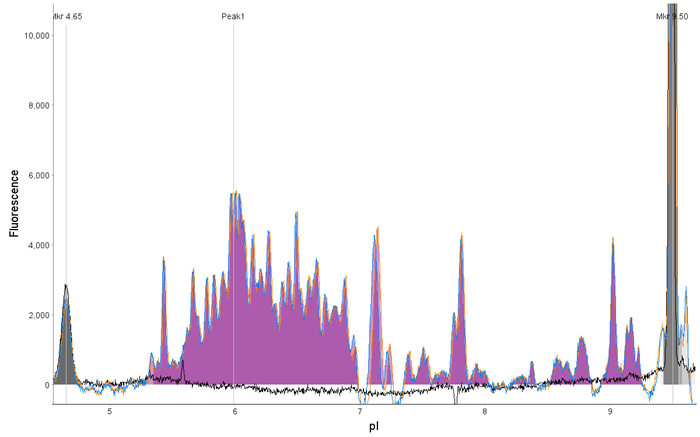
Figure 2. Analysis of LVV by CE-SDS and icIEF. LVV (1 x 109 TU/mL) was analyzed as described in the methods and compared to a blank by (A) CE-SDS Plus and (B) icIEF. Both methods offer rapid and unique fingerprints of the LVV capsid proteins. Image Credit: Bio-Techne
Turbo CE-SDS characterization of AAV capsid proteins
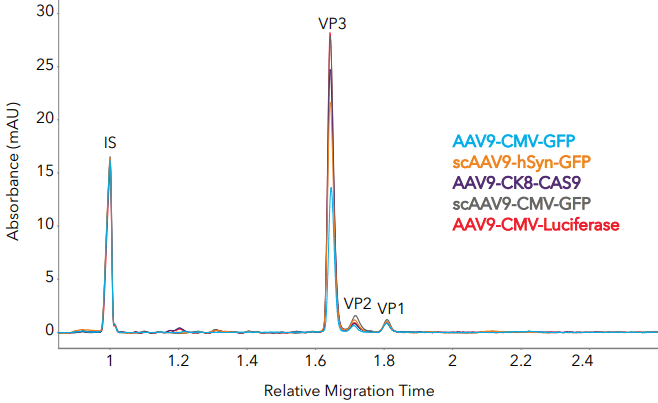
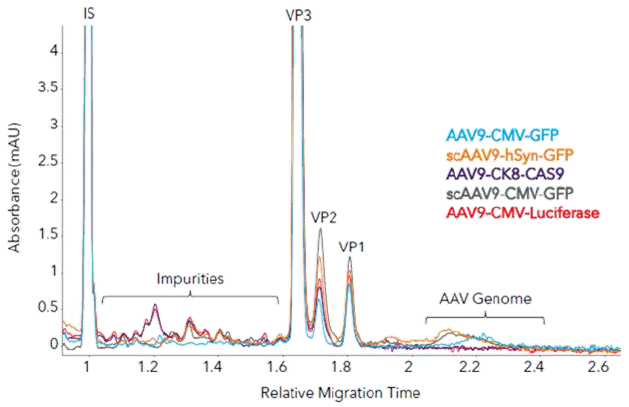
Figure 3. Measuring AAV capsid ratios and impurities in different AAV9 samples with Maurice Turbo CE-SDS. (A) Five AAV samples with different inserts were analyzed with Turbo CE-SDS and analyzed for capsid protein ratio and impurities. (B) Zoom-in to show impurities and AAV genomes. Image Credit: Bio-Techne
Table 2. AAV9 capsid protein ratios from Turbo CE-SDS and CE-SDS Plus. Source: Bio-Techne
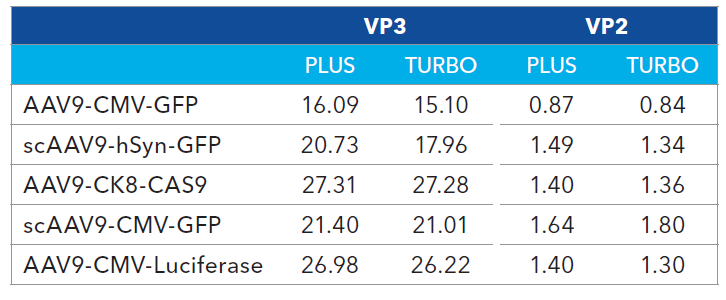
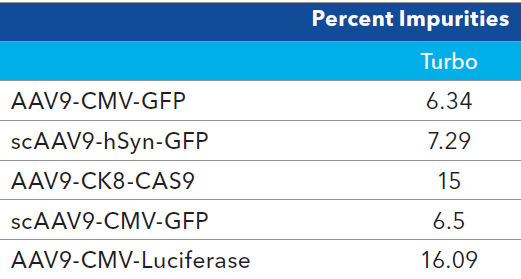
Table 3. Impurity analysis of AAV9 samples. Source: Bio-Techne
cIEF characterization of AAV capsid proteins
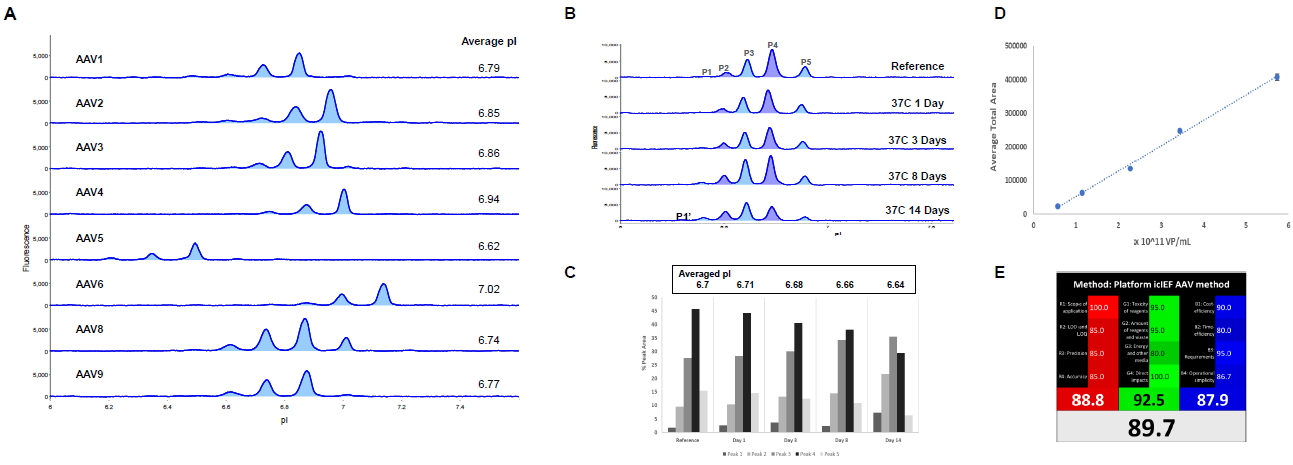
Figure 4. Imaged cIEF AAV Capsid Protein Method. (A) AAVs 1–9 were denatured prior to analysis by icIEF with native fluorescence detection (20s exposure). The averaged pI was determined from 3 injections of each AAV sample. (B) AAV8 (4.7 ×1011 VP/mL) was incubated at 37 °C prior to analysis. (C) Quantitation of AAV8 averaged pI (top panel) and %PA changes over time (bar graph). (D) AAV9 was denatured and then serially diluted two-fold from ~ 6 x 1011 VP/mL to 6 x 1010 VP/mL prior to analysis in triplicate on Maurice. A strong linear relationship was observed, with R2 > 0.99. (E) Evaluation of icIEF method for adherence to the principals of green/white chemistry standards, receiving an 89.7 % rating. See Wu and Heger, Green Analytical Chemistry 3 (2022), 100027 for more details.
MauriceFlex™ cIEF fractionation of AAV capsid proteins
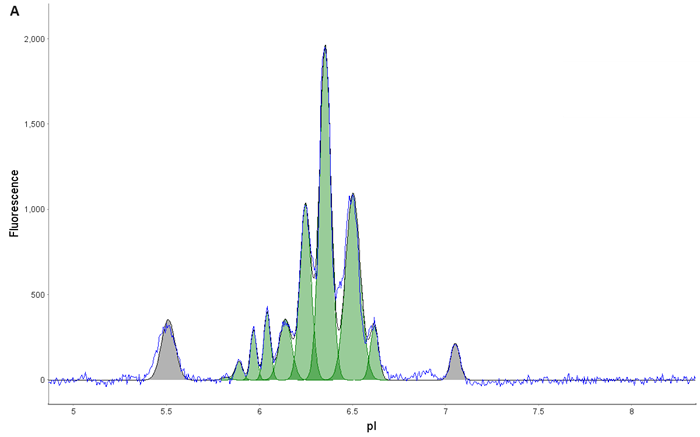
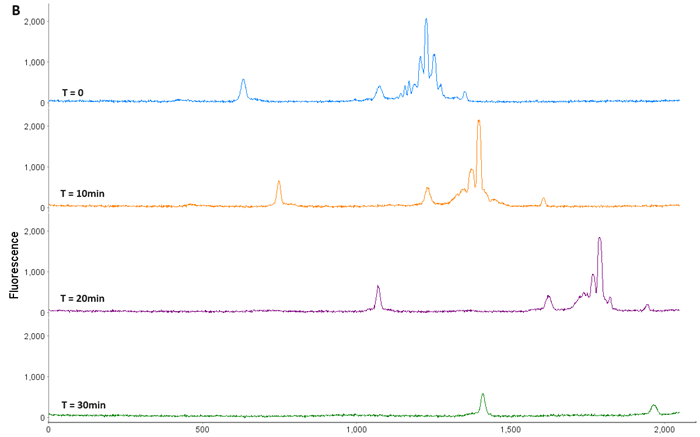
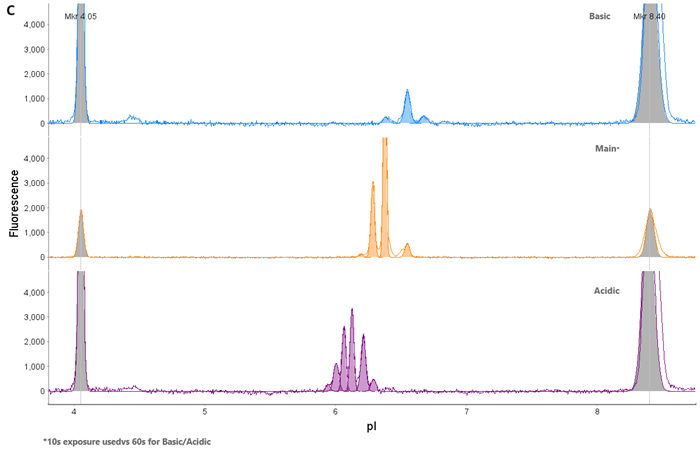
Figure 5. AAV9 Capsid Protein Fractionation. (A) AAV9 was loaded onto a Flex cartridge. (B) Capsid proteins mobilized out of the window within 30 min. (C) Fractions were checked on an icIEF cartridge to ensure isolation of individual acidic (VP1), main (VP3), and basic (VP2) species. Image Credit: Bio-Techne
Conclusions
MauriceFlex™ is a single platform that can be used to characterize various viral vectors used in cell and gene therapies via its CE-SDS, imaged cIEF, and charge-based fractionation capabilities.
Turbo CE-SDS delivers the AAV/LVV capsid protein ratio and purity analysis in less than 6 minutes per sample. Imaged cIEF is a potent stability-indicating identity assay for viral capsid proteins.
MariceFlex™ fractionation enables downstream characterization of charge variants via mass spectrometry and other techniques.
About Bio-Techne

Bio-Techne Corporation is a leading developer and manufacturer of high-quality purified proteins - notably cytokines and growth factors, antibodies, immunoassays, as well as biologically active small molecule compounds - which are sold to biomedical researchers and clinical research laboratories; these operations constitute the core Biotech Division, headquartered in Minneapolis, Minnesota.
The Protein Platform Division manufactures innovative protein analysis tools under the ProteinSimple brand name that greatly automate western blotting and immunoassay practices. The Diagnostics Division manufactures FDA-regulated controls, calibrators, blood gas, and clinical chemistry controls for OEM customers and clinical customers. Bio-Techne products assist scientific investigations into biological processes and the nature and progress of specific diseases. They aid in drug discovery efforts and provide the means for accurate clinical tests and diagnoses.
With thousands of products in its portfolio, Bio-Techne generated approximately $563 million in net sales in fiscal 2017 and has approximately 1,800 employees worldwide.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.