Sponsored Content by TissueGnosticsReviewed by Maria OsipovaNov 13 2025
Research published in Clinical and Translational Medicine offers insights into the colorectal cancer (CRC) immune macroenvironment. This recent study identifies key clinical biomarkers with the potential to support the stratification of CRC patients for immune checkpoint inhibitor (ICI) therapies.
CRC and the tumor macroenvironment
There were an estimated 1,926,136 cases of CRC in 2022, marking CRC as one of the most widely encountered cancer types.1 ICI-based neoadjuvant therapy has shown good therapeutic potential in the past 10 years.2
The tumor microenvironment (TME) is a bionetwork of stromal cells, immune cells, and extracellular components that organize the surrounding architecture and determine tumor progression and therapeutic response. The TME is key to the identification of important ICI responders in oncological treatments, but it is important to note that anti-tumor immunity is not limited to the TME.3
The tumor macroenvironment represents a far broader tumor environment which also responds to tumorigenesis, encompassing the primary tumor and surrounding organs, draining lymph node, spleen, bone marrow, and blood.
The tumor macroenvironment has displayed a shift in immune cells’ composition and function during tumor progression in mouse models, suggesting an alteration of the immune system.4
Atopic lymphoid tissues surrounding or within tumors are known as tertiary lymphoid structures (TLS). They are similar in function to secondary lymphoid organs like lymph nodes but develop ectopically to support immune responses at local sites of inflammation or disease. TLSs have attracted significant scientific interest because their presence and maturity can be linked to better patient outcomes in diseases like cancer, making them both potential biomarkers and therapeutic targets.5
Immune profiling methods for the CRC macroenvironment
Researchers in the published study explored variation within the CRC immune macroenvironment, looking at and noting fundamental immune macroenvironment-related influencing factors in response to ICI treatment in CRC patients.
A multi-disciplinary approach was employed, using a combination of multiomic techniques, including single-cell RNA-sequencing (scRNA-seq), cytometry by time-of-flight (CyTOF), spatial transcriptomics (ST-seq), and multiplex immunohistochemistry (mIHC) for immunoprofiling.
TissueGnostics’ TissueFAXS microscopy system enabled imaging of the mIHC-stained tumor and bowel sections and the capture of whole slide images. Quantities and distribution of marker-positive cells were then analyzed using the StrataQuest contextual image analysis software.
The use of cyTOF, scRNA sequencing, ST-seq, and immunofluorescent staining allowed immune cell heterogeneity to be characterized and validated. This highlighted variation in the CRC immune macroenvironment and linked important influencing factors in the immune macroenvironment to patient responses to ICI treatment.
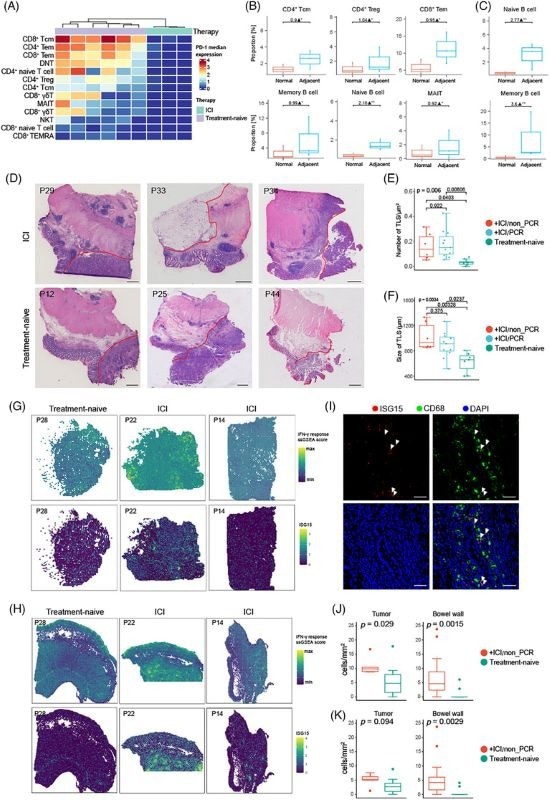
Image Credit: Figure 7. Immune profiling of the macroenvironment in colorectal cancer unveils systemic dysfunction and plasticity of immune cells by Ke et al, 2025
New insights into the CRC macroenvironment
Each bowel layer was determined to have distinct immune cell niches following the Immune profiling of non-cancerous human bowel samples.
For example, the epithelial layer saw enrichment of CD8+ Tem cells, CD8+ γδ T cells, CD8− γδ T cells, double negative T cells (DNT), and innate immune cells (Lin− CD7+), while mast cells, intermediate monocytes, CD16− NK cells, CD19+ or CD19− plasma cells, and natural killer T (NKT) cells were found to be enriched in the lamina propria.
Major immune cell types in the submucosa comprised CD4+ T cell subsets, including CD4+ naïve T cells, Tem, Tcm, and Treg. Naïve B cells, memory B cells, and group 3 innate lymphoid cells (ILC3) were also present. It was also noted that the muscularis propria was dominated by myeloid cell subsets.
In contrast, immune cell types documented in layers of tumor-adjacent bowel samples were notably different, displaying a tumor-specific phenotype. T cells were typically found to be terminally activated and exhausted when CD39+ CD4+ Tregs were increased in the marginal intestinal make-up. This had the potential to exacerbate tumor progression by promoting the formation of an immunosuppressive environment.
The CRC macroenvironment generally saw an increase in T cell immunosuppressive marker expression and a disturbance in immune cell composition.
Promising biomarkers for the CRC macroenvironment: SPP1–CD44, TLS, and CD69
An in-situ examination of the SPP1–CD44 interaction showed that only regions enriched by macrophages expressed SPP1 within the TME.
It was also determined that the SPP1-CD44 interaction identified in this study had a notable immunosuppressive effect directed by SPP1+ macrophages, marking this rewired interaction as a potential ICI therapy target.
TLS boosted anti-tumor immunity in the TME, with a higher TLS signature linked to improved overall survival rates in CRC patients. TLS presence also altered T cells’ functional capacity within the TME, while stromal cell enrichment was found to potentially inhibit TLS formation.
TLS was noted to be linked to the presence of CD8+ Tem cells in the PBMC, potentially serving as a predictive TLS biomarker within the CRC macroenvironment.
Additional analysis highlighted that the expression of CD69 and PD-1 in CD8+ Tem from PBMC represented promising prognostic markers for treated-naïve CRC patients.
Analysis of cell abundance revealed the enrichment of CD8+ T subsets, Lin− CD7+ cells, and DNT cells in consensus molecular subtype 1 (CMS1) tumors, as well as an increase in CD8+ T subsets and myeloid cell subsets in CMS3 tumors.
CMS2 tumors did not show a discrete immune profile, though CMS4 tumors exhibited a greater infiltration of naïve T cells and B cell subsets.
A study of PMBC immune cells in relation to the CMS group showed that reduced CD69 expression significantly distorted cells’ functional capacity in PMBC. CD69 was, therefore, regarded as a promising blood biomarker for the prediction of intrinsic CMS (iCMS) for CRC patients, potentially representing a novel means of selecting iCMS3 patients for ICI treatment.
The effects of ICI treatment on the CRC macroenvironment
CRC patients receiving ICI treatment have been shown to exhibit increased IFN-γ response pathway activation in the stromal region in bowel wall or tumor samples.
Tumors with ICI treatment showed high expression of the ISG15 gene, with ISG15+ cells found to increase in both the tumor and normal bowel wall. However, ISG15+ CD68+ macrophages were only found to increase in the normal bowel wall. This was revealed via quantitative image analysis in StrataQuest.
It was determined that ICI treatment prompted a systematic IFN-γ response through TLS formation, improving CD8+ T cell expansion. In contrast, ISG15 was characterized as a proinflammatory marker linked to poor response to immunotherapy in CRC patients.
Conclusion
This study’s findings offer fundamental insights into the CRC macroenvironment, revealing several distinct immunotypes. TLS presence is positively associated with ICI efficacy, CMS cell enrichment, and overall patient survival rates.
The strategic combination of powerful multiomic technologies, including the TissueFAXS slide scanning platform, was key to enabling the identification of vital biomarkers, allowing the researchers to gain valuable insight into the utility and implementation of the immune macroenvironment in clinical decision-making for CRC patients.
References and further reading
- Siegel, R.L., Giaquinto, A.N. and Jemal, A. (2024). Cancer statistics, 2024. CA: A Cancer Journal for Clinicians, 74(1), pp.12–49. https://doi.org/10.3322/caac.21820.
- Cercek, A., et al. (2022). PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. New England Journal of Medicine, 386(25). https://doi.org/10.1056/nejmoa2201445.
- Zeng, D., et al. (2021). Tumor microenvironment evaluation promotes precise checkpoint immunotherapy of advanced gastric cancer. Journal for Immunotherapy of Cancer, (online) 9(8), p.e002467. https://doi.org/10.1136/jitc-2021-002467.
- Allen, B.M., et al. (2020). Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nature Medicine, 26(7), pp.1125–1134. https://doi.org/10.1038/s41591-020-0892-6.
- Ogino, S., et al. (2009). Lymphocytic Reaction to Colorectal Cancer Is Associated with Longer Survival, Independent of Lymph Node Count, Microsatellite Instability, and CpG Island Methylator Phenotype. Clinical Cancer Research, (online) 15(20), pp.6412–6420. https://doi.org/10.1158/1078-0432.CCR-09-1438.
- Ke, H., et al. (2025). Immune profiling of the macroenvironment in colorectal cancer unveils systemic dysfunction and plasticity of immune cells. Clinical and translational medicine, (online) 15(2), p.e70175. https://doi.org/10.1002/ctm2.70175.
Acknowledgments
Produced from materials originally authored by TissueGnostics.
About TissueGnostics
TissueGnostics (TG) is an Austrian company focusing on integrated solutions for high content and/or high throughput scanning and analysis of biomedical, veterinary, natural sciences, and technical microscopy samples.
TG has been founded by scientists from the Vienna University Hospital (AKH) in 2003. It is now a globally active company with subsidiaries in the EU, the USA, and China, and customers in 30 countries.
TissueGnostics portfolio
TG scanning systems are currently based on versatile automated microscopy systems with or without image analysis capabilities. We strive to provide cutting-edge technology solutions, such as multispectral imaging and context-based image analysis as well as established features like Z-Stacking and Extended Focus. This is combined with a strong emphasis on automation, ease of use of all solutions, and the production of publication-ready data.
The TG systems offer integrated workflows, i.e. scan and analysis, for digital slides or images of tissue sections, Tissue Microarrays (TMA), cell culture monolayers, smears, and other samples on slides and oversized slides, in Microtiter plates, Petri dishes and specialized sample containers. TG also provides dedicated workflows for FISH, CISH, and other dot structures.
TG analysis software apart from being integrated into full systems is fully standalone capable and supports a wide variety of scanner image formats as well as digital images taken with any microscope.
TG cooperations
TG continuously cooperates with research groups and other companies in the industry to provide novel tools and applications to its customers.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.