An interview with Ariel Louwrier, Ph.D., discussing the development of pre-formed fibrils for research into neurodegenerative disorders such as Alzheimer's and Parkinson's disease.
What do we know about the roles of Tau and Alpha-Synuclein in the healthy human brain?
Unfortunately, little is known about this subject. We know that tau is involved in microtubule formation, which allows a supply of goods to reach cells and keep them healthy. In Alzheimer's disease, tau becomes hyper-phosphorylated, causing neurofibrillary degeneration and subsequently, cell death.
We know that alpha-synuclein, a monomer building-block protein, is abundant in the brain. It exists in a variety of forms including oligomers, fibrils, and larger deposits in patients suffering from Parkinson’s disease or multiple systems atrophy (MSA).
Alpha-synuclein accumulates to form Lewy bodies or Lewy neurites, which can be circular or elongated in structure. We believe that these Lewy bodies are toxic and involved in the killing of dopaminergic neurons, leading to the development of movement disorders like Parkinson's disease.
Overall, these are very complicated proteins that can form multimers with each other. They are dependent on phosphorylation states and other post-translational modifications, which can be quite complex.
 Image Credit: medistock / Shutterstock.com
Image Credit: medistock / Shutterstock.com
How can active pre-formed fibrils be used to study Alzheimer’s and Parkinson’s disease?
Pre-formed fibrils are tools that allow scientists to mimic the pathophysiological effects of Alzheimer's and Parkinson's disease in the brain. It was hypothesized that recombinant fibrils could be synthesized and introduced to cells to induce the pathology of the disease, allowing a model to be developed.
Pathologies can be induced by over-expressing tau or alpha-synuclein endogenously, or by using tau or alpha-synuclein mutants that aggregate more rapidly. However, the transgenic animals needed for these experiments can take a long time to mature, meaning investigations can be lengthy processes. In contrast, injecting fibrils can induce pathology in as little as 30 days.
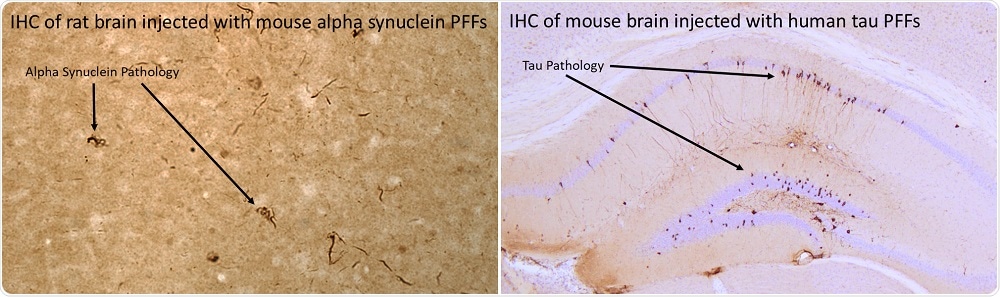
(L) Mouse alpha-synuclein PFFs induce alpha-synuclein pathology 30 days post-injection into rat brain. (R) Human P301L mutant K18 PFFs induce tau pathology nine weeks post-injection into P301L transgenic mouse brain.
As the fibrils are recombinant in nature, this generates both opportunities and challenges as they are not exact replicas of the structures found in the brain. For instance, if we express fibrils in E. coli., the host does not have the molecular machinery to carry out translation modifications that, in some instances, can be extremely important and are an inherent part of these proteins in humans. Conversely, the fact that these differences exist allows clear differentiation of endogenous and exogenous proteins.
We have shown that fibrils are not only taken up, for instance, by neurocortical primary cells, but can also be transported within these cells. This is essential for inducing the pathology associated with Parkinson's, which is detected using a phospho-antibody to one particular amino acid chain at position 129.
Fibrils can be injected into cell culture or directly into the rodent brain; allowing us to identify and measure the pathology, and giving scientists a model to test new drug candidates.
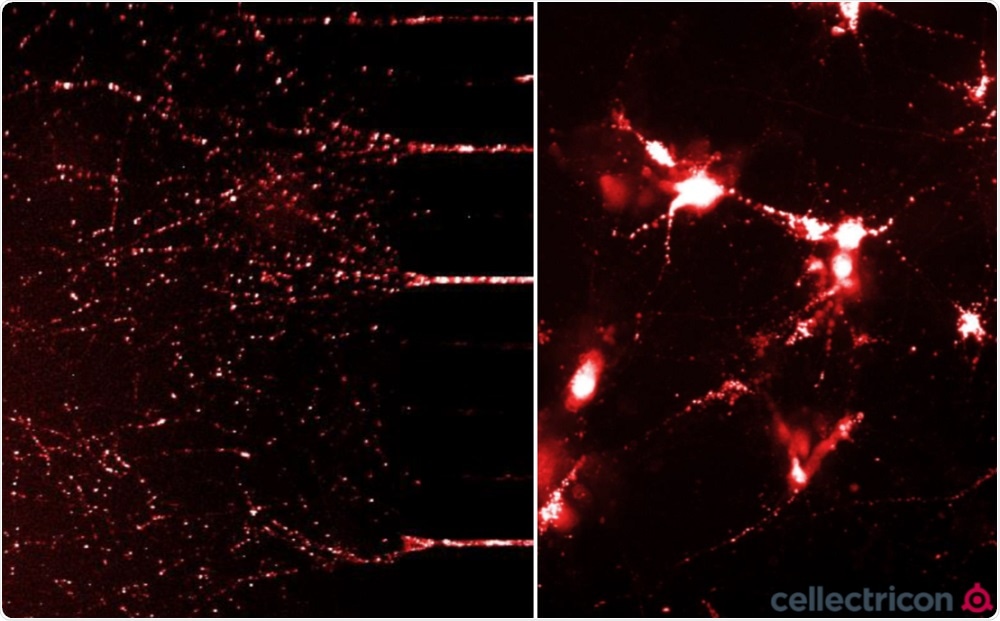 ATTO633 fluorescently-labelled alpha-synuclein PFFs were taken up, transported into the soma, and induced alpha-synuclein aggregation in mouse neurocortical primary cells. (L) Neurites filled with fluorescently-labelled alpha-synuclein seeds in a microfluidic co-culture system after 24 hours. (R) Alpha-synuclein seeds within the soma and neurites of mouse neurocortical primary cells after 24 hours. Experiment and imaging courtesy of Cellectricon.
ATTO633 fluorescently-labelled alpha-synuclein PFFs were taken up, transported into the soma, and induced alpha-synuclein aggregation in mouse neurocortical primary cells. (L) Neurites filled with fluorescently-labelled alpha-synuclein seeds in a microfluidic co-culture system after 24 hours. (R) Alpha-synuclein seeds within the soma and neurites of mouse neurocortical primary cells after 24 hours. Experiment and imaging courtesy of Cellectricon.
What other methods are available for inducing neurodegeneration in vivo? How do these methods compare to pre-formed fibrils and oligomers?
One way is to introduce alpha-synuclein at higher levels genetically. Alternatively, alpha-synuclein or tau can be introduced via virus invasion. Through those mechanisms, you can also introduce mutated versions that are more aggressive and accelerate the aggregation process.
We use a similar concept with the fibrils. For instance, one of the mutations in familial Parkinson's disease is at amino acid position 53. We make A53T mutant fibrils, which are much more aggressive in seeding aggregation in vitro and in vivo than the traditional wild-type fibrils. The use of fibrils is faster and more sophisticated than genetic overexpression or viral vector methods.
Our aim is to introduce something that is as close as possible to what is in the brain. Cells help us with this. One research group recently found that the introduced fibrils can be hydrolytically processed - cleaved - after entering the cells and can undergo post-translation modifications as well.
With a similar goal in mind, we have made a tau construct that is phosphorylated and post-translationally modified using F9 cells. It fibrilizes independently without induction. This product will be launched very soon. We are very interested to see its uptake, how it is used, and what the results are.
Fibrils tend to form very specific structures that look like helices. They are two entities twisted around each other, called a paired helical filament, although single twisted strands also exist. We have grown some of these filaments on natural, removable linear scaffolds.
 Image Credit: Design_Cells / Shutterstock.com
Image Credit: Design_Cells / Shutterstock.com
Where did the company start? How were these products developed?
The efforts relating to the generation of tools for neurodegenerative disease research started with an email inquiry from a researcher on the East Coast (USA). She had been forwarded our details by another academic for whom we had made a few other constructs, in an area that had essentially nothing to do with neuroscience.
The researcher wanted to see if we could make pre-formed alpha-synuclein fibrils. As a protein chemist, I thought this would be simple. There are not many companies that will take a risk and try things without charging for it, but we are one of them.
We made the construct and sent it to the researcher, but they came back and said that it did not work. It took us six or seven months to figure out that we had made an entirely different type of fibril than what they had been expecting. This was a huge learning curve for us because the literature overwhelmingly points to a single type of fibril on which most of the research is based.
Our studies suggested that there are at least two types of fibrils, and that they are very different from each other. There is some literature to support this, but researchers normally assume that there is one type that sometimes works and sometimes does not. However, the aim of researchers is generally not to make the tools or the fibrils.
They are far more interested in assessing downstream events once the fibrils have been introduced into a living system. This would include attempting to neutralize the generation of pathology using their own drug candidates. However, the fibril type is critical as you cannot neutralize a pathology that has not been generated in the first place!
We began to realize that not only was there an opportunity from a commercial perspective, but there was also something scientifically important that has been missed that we could provide to the research community. That led us down this path of development, and since then we have made multiple different types of fibrils for different applications, as well as supplying the monomers.
Most importantly, there has never been a good control for any of this work. When no other fibril is available, people traditionally use monomers. This potentially creates an issue, as monomers will self-nucleate and start to fibrilize on their own, so whilst they are fine for a short-term experiment, they can be unsuitable for a long-term study.
To solve this, we developed a method to force a very close cousin of alpha-synuclein, called beta-synuclein, to fibrilize. It is a totally inert, completely artificial construct that does not seed alpha-synuclein or itself. This is the first time that there is a fibrillar control available for any industry. On top of this, we have made antibodies to these constructs.
Why did you feel it was important to add dopamine-stabilized oligomers to your portfolio?
There are limitations to the utility of fibrils. Many of them are poorly understood because they are insoluble. The major players in the field, such as the Michael J. Fox Foundation, which does an unbelievable job in the area, assert in their publications that fibrils require sonication to bring them down to a certain size (length) in order to be used in experiments.
Sonication is a violent process. It involves the creation of microbubbles that collapse amid temperatures that we would generally associate with the center of the sun - one to two million degrees centigrade. Clearly, the results are that there is a tremendous amount of oxidative stress going on, something that is often not considered when these fibrils are used.
Furthermore, what we know about alpha-synuclein revolves around dopamine-generated cells. So, we wanted to be able to look “earlier” into the process before fibrils are made, when the monomers make up soluble oligomers – a different form of the aggregated protein to fibrils.
There is a general belief in the industry that oligomers are the toxic species. Dopamine-stabilized oligomers were the first to be manufactured and launched because they are thought to be more biologically relevant than other types.
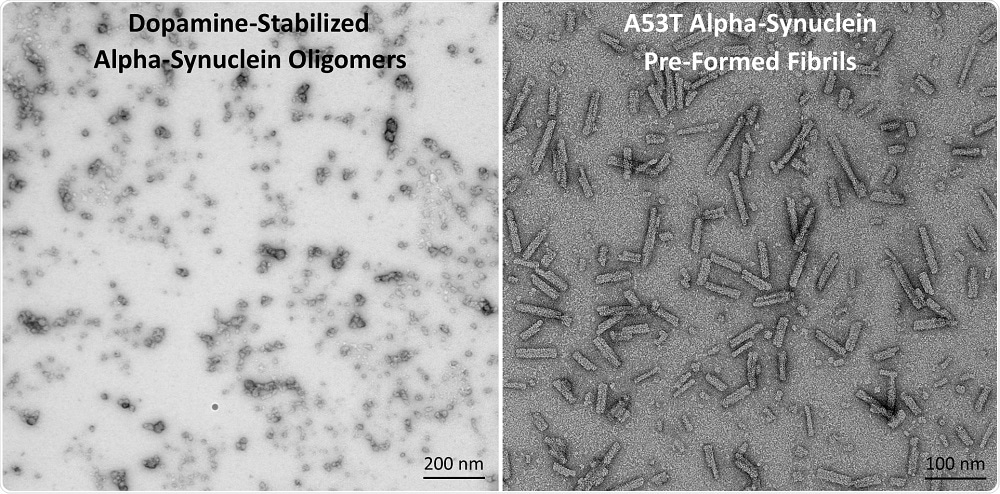 (L) TEM of Dopamine-Stabilized Alpha-Synuclein Oligomers (R) TEM of A53T Alpha-Synuclein PFFs.
(L) TEM of Dopamine-Stabilized Alpha-Synuclein Oligomers (R) TEM of A53T Alpha-Synuclein PFFs.
How should the pre-formed fibrils be prepared for use? Why is sonication important here?
It depends on the construct being used. Generally speaking, the two methods needed to make fibrils are purification and shaking. First monomers are purified, and then the environment becomes critical, as this determines what happens next. Monomers need to be shaken for days, which accelerates the fibrillization process.
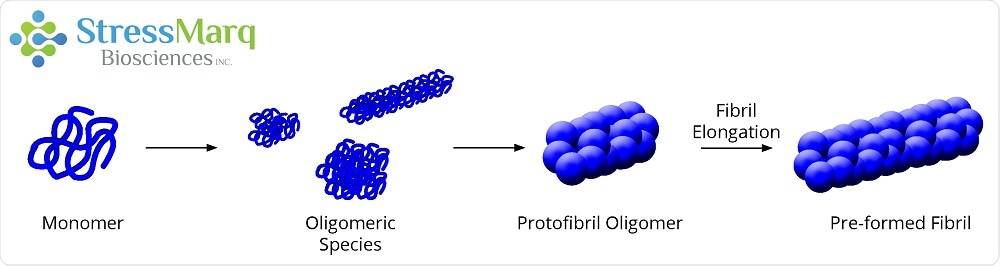 Monomers aggregate to form oligomers, protofibril oligomers, and ultimately lengthen into fibrils.
Monomers aggregate to form oligomers, protofibril oligomers, and ultimately lengthen into fibrils.
The nuances in these methods are very important; for instance, which scaffold is being used? If the scaffold cannot be removed, will that introduce another factor and accelerate more generation of that particular construct outside of your control?
As I mentioned earlier – sonication is often thought of as being necessary as a method that allows reduction of fibril size to a level where it is thought to be able to generate pathology. We think this is probably an artefact of what is really relevant, which is solubilization of the fibrils. This occurs during sonication. Once phase differences (soluble and insoluble) have been removed between monomeric and fibrillar species, any diffusional limitations associated with those two phases are reduced or removed.
Why is it important to select pre-formed fibrils from an appropriate species?
Ultimately most people want to work with human constructs. However, people do work in rodents at an earlier stage. Rodents are well characterized and there are many transgenic constructs available.
It may also make more sense for researchers to put a mouse construct into a mouse rather than a human construct into a mouse. The ultimate experiment has just one variable, and whilst this is not always possible in vivo, it is what researchers are trying to achieve and this allows them to be one step closer to that paradigm.
Having more than one construct to choose from can be very useful because some versions are more aggressive. There are, for instance, human constructs that contain mutations to make them more like the mouse construct, which is more aggressive than a human construct. Ultimately, researchers generally want to move in the direction that gives them data relevant to the human condition.
We collaborated with the CRO to test human and mouse constructs in mice. With the mouse constructs, pathology can be seen not only in the injection site but everywhere in the brain in all cases after 30 days. This is important as it shows that the pathology can spread.
In contrast, however, when you use the human version, the seeding is less aggressive and so the pathology develops at a much slower rate. After injection with human constructs, pathology develops in about 25 percent of the rats after 30 days compared to 100 percent of the rats injected with the mouse construct. In some applications, time is important, and this is when using a quicker method of pathology generation may be appealing.
What other pre-formed fibrils and monomers do you offer?
We have a number of other fibrils and monomers including transythyretin (TTR) and superoxidase dismutase 1 (SOD1). In terms of tau, we also have different versions and sizes.
There is another protein of interest called TDP-43 that is important in Alzheimer’s disease. That is a difficult one that we have not been able to successfully make yet.
In some cases, the formation of oligomers or fibrils can go in a multitude of directions, generating different types of constructs that have varying properties, depending on synthesis conditions. Standardizing these methods as well as the construct characterization is part of the challenge we face.
Are you planning to expand the product range? If so, which proteins will you be focusing on next and why?
Yes, developing new constructs is an ongoing process. We want to be able to discriminate between fibrillar constructs, for instance, such as between the two types of alpha-synuclein fibrils, so we are developing antibodies to do that.
In the immediate future, one of the principal efforts is going to be with oligomers. We have launched the dopamine oligomers, which has caused the fastest uptake of any new products in our history.
We also want to make other versions that are induced by physical components rather than covalent modifications. In addition, we want to make constructs that are more artificial and induced by small molecules.
Ultimately, we want to create something that is as stable as possible that mimics what happens in real life; at the moment we do not know which construct that is. It may well be the dopamine option, but determining that is in the domain of Parkinson’s disease researchers. We will make, introduce, and provide various oligomers. Some of this will rely on collaboration with other organizations, which is something we are always keen to do.
We plan to make antibodies for all constructs in order to differentiate them. At the moment, we have one that seems to identify oligomers. We have done several projects on these constructs and have screened hundreds of candidates, but we have only one antibody so far. This is another area where we see potential.
Oligomers are the way forward and they have proved to be a tricky endeavour. They need to be made and properly characterized, and any given synthesis may generate more than one population. Perhaps we will be able to provide more than one population of oligomers soon – this is part of our aim and commitment to the research sector.
Where can our readers find more information?
About Ariel Louwrier, Ph.D.
 Ariel Louwrier is the President and CEO of StressMarq Biosciences Inc., a life sciences company based in Victoria, Canada. The company specializes in research products for neurodegenerative disease research. Ariel holds a Ph.D. in Biochemistry from the University of Kent (UK), and is a Corporate Fellow of the Cell Stress Society International.
Ariel Louwrier is the President and CEO of StressMarq Biosciences Inc., a life sciences company based in Victoria, Canada. The company specializes in research products for neurodegenerative disease research. Ariel holds a Ph.D. in Biochemistry from the University of Kent (UK), and is a Corporate Fellow of the Cell Stress Society International.