In the current COVID-19 pandemic, drug repurposing is being widely investigated as a way to find treatments rapidly. Now, a new study addresses the possible use of an FDA-approved monoclonal antibody, called fostamatinib, a spleen tyrosine kinase inhibitor (SYK), to reduce the levels of mucin-1, a molecule associated with acute lung injury and acute respiratory distress syndrome (ARDS). People who have severe COVID-19 disease may develop ARDS.
The research titled ‘A High Content Screen for Mucin-1-Reducing Compounds Identifies Fostamatinib as a Candidate for Rapid Repurposing for Acute Lung Injury during the COVID-19 pandemic’, was published on the preprint server bioRxiv* in June 2020.
Drug Repurposing
When an approved or experimental drug is used for a condition that falls outside the range of the purpose for which it was originally approved, it is said to be repurposed. This strategy is being intensively investigated in the current pandemic because of the absence of any antiviral or vaccine that is effective against SARS-CoV-2, the virus causing COVID-19.
Drug repurposing has a much lower risk of toxicity compared to newly developed drugs as they have already passed safety trials. Secondly, they take a much shorter time to produce on market scale as several stages of drug development are already complete.
Currently, supportive and intensive medical management is all that can be offered to COVID-19 patients. A significant percentage of patients suffer severe respiratory distress and acute lung injury, with high mortality rates, especially if certain medical conditions are already present. As of now, over 500,000 people have already succumbed to the virus in just six months, which leaves healthcare workers urgently seeking new drugs to treat very ill COVID-19 patients effectively.
Reducing Mucin-1 Levels
Mucin-1 is a transmembrane glycoprotein secreted from most of the mucosal epithelium. It is an essential protein in restricting the size of the airway passage. Goblet cells can rapidly secrete mucus which is exocytosed to form a mucus layer over the epithelial lining of the airways when stimulated by specific triggers.
In a healthy person, mucus in the airways protects the lungs from inhaled bacteria, fungi, and viruses, as well as toxins and dust particles, but too much mucus can sharply reduce the lumen available for air to pass. This is therefore associated with more frequent and prolonged infections, impairment of lung function, and a higher number of deaths after a respiratory infection.
High mucus production is also linked to lung disease and death in conditions like cystic fibrosis and chronic obstructive pulmonary disease (COPD). As a result of these findings, the current study looked at drugs that could reduce the levels of this protein.
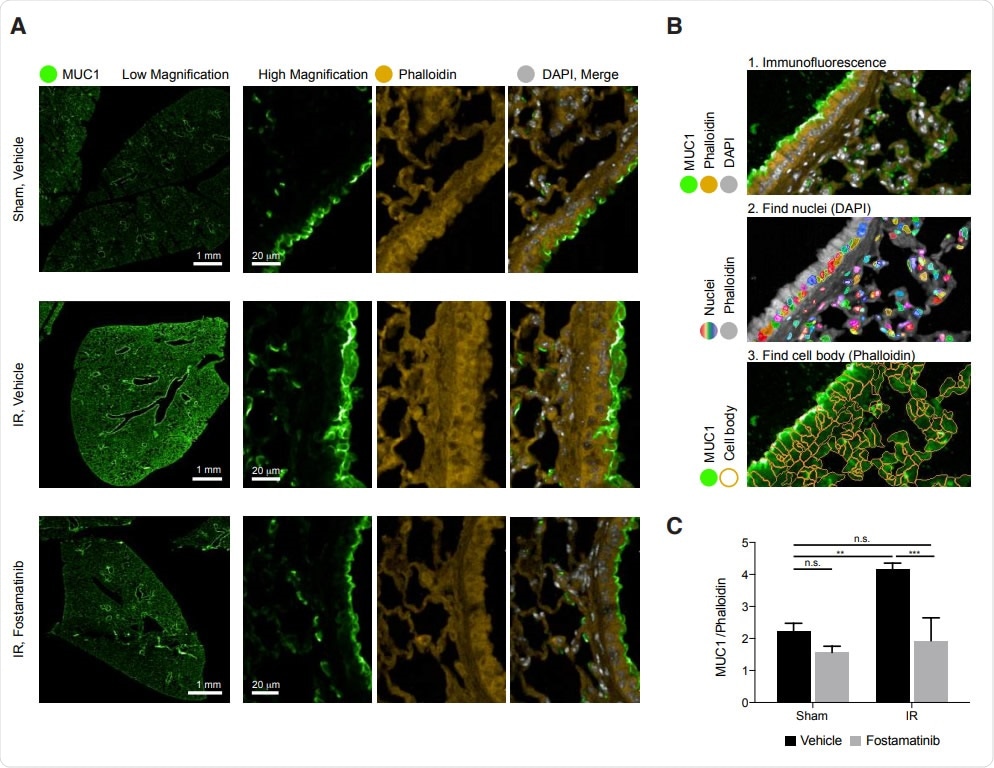
In vivo, R788 reduces excess MUC1 from lung epithelia of mice with AL

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The Search for the Right Repurposed Drug
The criteria for an acceptable repurposed drug were:
- Dose-dependent reduction of mucin-1
- Favorable toxicity profile
- Non-transcriptional mechanism of action, since transcriptional inhibitors like vitamin D agonists, did not work in clinical situations
- FDA-approved
From over 3,700 compounds, they narrowed down four compounds, which reduced mucin-1 production but did not affect mRNA nor produced toxic effects.
The researchers identified the prodrug fostamatinib, which is rapidly converted to the active metabolite R406, as a potential compound for repurposing. They found that this decreased the levels of mucin-1 from epithelial cells while preserving cell viability. The control molecule was the bromodomain inhibitor JQ1, which completely suppresses transcription of mucin 1.
Previous Research Findings
Experiments on animal models have shown this drug to be effective in severe inflammatory conditions such as immune glomerulonephritis or vasculitic disorders. It is currently undergoing phase II trials to evaluate the effect of SYK suppression in an inflammatory renal disorder called IgA nephropathy.
The prodrug fostamatinib was approved for the treatment of chronic immune thrombocytopenia and has also been used safely to treat over 3,000 patients with rheumatoid arthritis. Its safety profile commends it to researchers looking for COVID-19 therapies.
R406 is the active metabolite of fostamatinib. This is an SYK inhibitor that acts by dephosphorylation of integral membrane proteins, after which they are removed from the plasma membrane by endocytosis. Apart from mucin-1, it also regulates the abundance of the gel-like mucin MUC5AC in the human nasal epithelium, and human mucin-producing cells. And finally, the deletion of the Muc1 gene in airway epithelial cells in rats and human lung epithelium causes reduced hypersecretion of mucin, which in turn protects against lung injury.
Actions of R406
R406 was found to deplete mucin from the plasma membrane and redistribute a part of it to the inside of the cell, mostly around the nucleus. Earlier studies showed that SYK inhibition suppresses both local and remote lung injury.
The researchers supplied a compound called fostamatinib disodium or R788, a methylene phosphate prodrug of R406, that can be orally ingested, to mice and studied the lung tissue. They found that ischemic-reperfusion injury caused acute lung injury, which in turn led to high levels of mucin-1 in the lung epithelium. They concluded that this is “consistent with previous reports that excess MUC1 is injurious.”
However, when they examined the lung tissue of mice treated with R788, they found significantly reduced mucin-1, as seen by immunohistochemical imaging and quantitative image analysis.
Mechanism of Action of R406
The study thus shows that R406 could be used to reduce mucin-1 levels in the lung epithelium in patients with acute lung injury. This molecule powerfully inhibits SYK, which is a protein tyrosine kinase in the cell cytoplasm, mediating the expression of multiple inflammatory chemicals. Most leukocytes express SYK, and it plays a role in immune signaling pathways, whether through B cell receptors or Fc receptors.
SYK is also involved in cellular adhesion, mediates recognition of antigens by innate immune cells, and in the activation of platelets. Its key role in immune regulation means that many drugs target this enzyme, including over 50 SYK small molecule inhibitors to treat a range of conditions, from asthma to arthritis.
Recent studies show that KL-6/MUC1 levels are reliable prognostic biomarkers of disease severity in patients with COVID-19-related acute lung injury.
Therefore, say the researchers, “Given the roles of excess KL6/MUC1 in ALI and ARDS, we propose that Fostamatinib may confer benefit in patients with COVID-19 lung injury.”
They suggest that this provides a foundation for future clinical trials which will test the usefulness of repurposing this drug for COVID-19 patients.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources