Using mouse models, scientists have investigated the active plasma metabolite of remdesivir, GS-441 524, and hydroxychloroquine as possible drug candidates in COVID-19. The research shows GS-441 524 could be an effective therapeutic drug against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), while at the same time discounting hydroxychloroquine as a reliable anti-viral.
Scientists worldwide are trying out a plethora of existing drugs to neutralize SARS-CoV-2 or treat COVID-19 disease, and remdesivir and hydroxychloroquine were identified early on in the pandemic as promising therapeutic options.
While hydroxychloroquine is a polymerase inhibitor classically used as an anti-malarial medication, remdesivir is a broad spectrum anti-viral prodrug. GS-441 524 is the resulting major circulating plasma metabolite of remdesivir, and its pharmacokinetic and physical-chemical properties are little known. Direct administration of GS-441 524 may prove to be effective and advantageous. To find out this possibility, as a first step, clinical in vivo animal-model tests to investigate the drug's pharmacokinetics and pharmacodynamics are required.
In a recent bioRxiv* paper, Oliver Scherf-Clavel et al. demonstrate the suitability of the drugs GS-441 524 and hydroxychloroquine against SARS-CoV-2. Using mouse models, they investigate the plasma concentration and distribution of the drugs in relevant tissues. They have demonstrated that the mouse model platform is suitable to characterize the pharmacokinetics of the drugs. A useful tool is presented in this study for an early investigation of drug candidates targeted against SARS-CoV-2. It is observed that both the drugs, GS-441 524 and hydroxychloroquine, are significantly distributed into the examined tissues compared to the plasma.
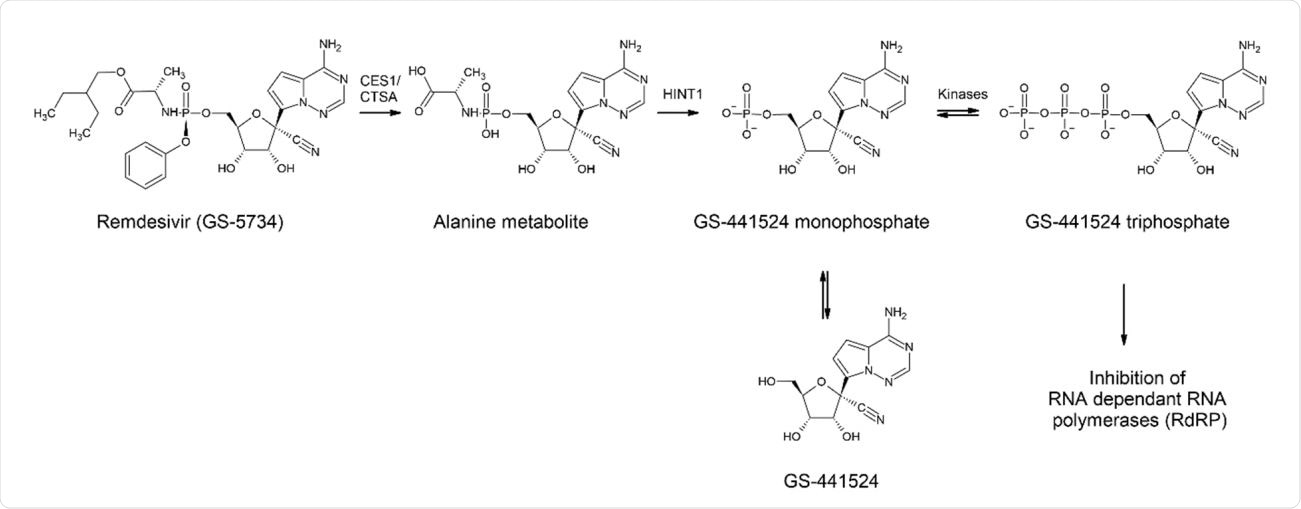
Metabolism of REM (GS-5734) leading to GS-441 524

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
A prodrug is used as a strategy to improve physicochemical, biopharmaceutical, or pharmacokinetic properties of pharmacologically potent compounds and thereby to overcome barriers to a drug's developability and usefulness. In this study, the researchers show that though remdesivir is not administered as a prodrug, its active metabolite GS-441 524 is detected in tissues. Mainly, a ten-fold increase in concentration is observed in vivo compared to in vitro, in the case of GS-441 524. However, comparatively, hydroxychloroquine did not show promising results.
GS-441 524 is already used as an effective drug against feline infectious peritonitis - a fatal immune-mediated infectious disease in cats caused by a 'feline coronavirus'. Similarly, researchers consider GS-441 524 to be effective against SARS-CoV-2 in vivo. In the case of hydroxychloroquine, due to slow and insufficient tissue saturation, it may be excluded for effective anti-viral treatment.
Significance of this study
While isolation and supportive care (including oxygen therapy, fluid management, and antibiotics treatment for secondary bacterial infections) are recommended to COVID-19 patients, the disease is rapidly progressing in some cases to severe conditions, including septic shock and multiple organ failure. Initial management of COVID-19 is thus crucial; to date, 30.9 million cases of COVID-19 have been confirmed worldwide.
With this ongoing pandemic, scientists are vigorously testing diverse therapeutic options. Exhaustive in vivo animal studies are mandatory. Also, to calculate the appropriate dose of a drug, the relationship between pharmacodynamics and pharmacokinetics needs to be assessed. This study may throw significant possibilities of new or repurposed drugs in humans against SARS-CoV-2.
The authors explain in this paper that the theoretical considerations show remdesivir to be an inappropriate strategy for the treatment of COVID-19. Remdesivir was explicitly designed as a prodrug to target the Ebola virus. The COVID-19 patients exhibit a broad and extensive multi-organ pathology, which may benefit directly with the drug GS-441 524. The latter is detected in exceedingly high concentrations in all measured organs except CNS (central nervous system) and fat. In this study, the researchers highlight an important question: whether or not the foci of infection could be reached, in vivo, by the drug under study – GS-441 524 and hydroxychloroquine.
They investigated the plasma half-life for GS-441 524 and the origin of GS-441 524 after administrating it as a phosphoramidite prodrug. The pharmacokinetics of GS-441 524 in this study compared to the pharmacokinetics observed in cats; however, increased clearance and reduced volume of distribution is found. This highlights the importance of pharmacokinetics in species to model COVID-19.
Although initial studies look promising, further investigations are needed for the therapeutic possibilities of the drug: reduction in SARS-CoV-2 viral load or significant clinical benefit, reduced time of viral shedding, reduced time to alleviation of symptoms, and reduced hospital stay.
This study indicates a prerequisite that points: GS-441 524 could indeed be effective against SARS-CoV-2 in vivo.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Sources:
Journal reference:
- Preliminary scientific report.
Mouse model for testing SARS-CoV-2 anti-virals: Pharmacokinetics, Oliver Scherf-Clavel, Martina Kinzig, Bettina Friedl, Edith Kaczmarek, Malte Feja, Manuela Gernert, Franziska Richter, Rainer Hoehl, Roland Nau, Ulrike Holzgrabe, Fritz Soergel, bioRxiv 2020.09.16.299537; doi: https://doi.org/10.1101/2020.09.16.299537