Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a novel RNA virus from the betacoronavirus family. Members of the coronavirus family have adapted to human hosts and effectively get transmitted from person to person via the respiratory route. Seven different coronaviruses with varying incubation periods, transmissibility, and disease severity have affected humankind so far. They are, in the order of their mortality rate, MERS‐CoV > SARS‐CoV > SARS‐CoV‐2 > HKU1 ≃ NL63 ≃ OC43 ≃ 229E.
Of these, SARS‐CoV‐2 is unique and has a relatively long incubation time. Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 emerged in Wuhan, China, and has now progressed into a pandemic of unprecedented proportions, causing significant morbidity and mortality worldwide. Apart from its ability to cause acute respiratory failure, this virus is also capable of triggering disturbances in hemostatic balance, which is a significant problem in patients with moderate and severe disease. These disturbances can lead to hypercoagulability and disseminated intravascular coagulation (DIC), causing organ failure, heart and kidney complications, and stroke.
In recent weeks, blood clotting disorders have surfaced as one of the major complications in severe COVID-19 patients. Remarkably, over 70% of deaths associated with COVID-19 are attributed to clotting-related complications, including pulmonary embolism, multi-organ failure, and strokes, which have been confirmed by autopsy.
In a study by researchers from the University of South Carolina School of Medicine, The Pennsylvania State University, and Postgraduate Institute of Medical Education & Research, Chandigarh, India, explored the possible mechanisms of the clotting disorder, focusing on the role of hypoxia-related activation of coagulation factors; cytokine storm, activation of neutrophils, and the release of neutrophil extracellular traps. This study is published in the journal Reviews in Medical Virology.
In the study, the researchers briefly reviewed how multifactorial pathologies and molecular responses affect the coagulation system and the fibrinolytic system in COVID‐19 patients.
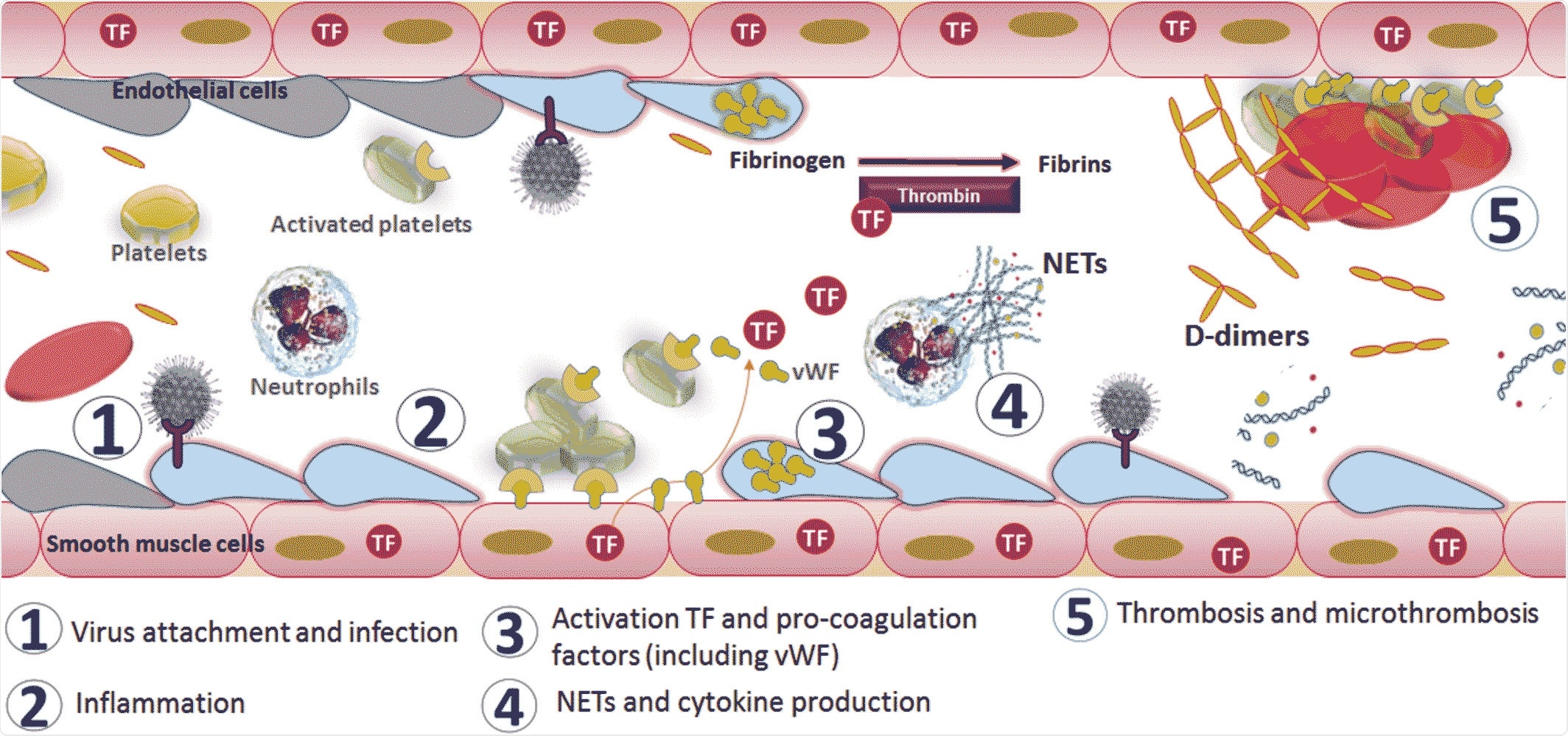
Mechanism of activation of coagulopathy in severe acute respiratory syndrome coronavirus 2 infection. Image Credit: John Wiley & Sons, Inc.
‘Cytokine storm’ leads to destructive inflammatory responses in severe COVID-19
A typical SARS‐CoV‐2 infection runs for about 20 days. The initial infection manifests a set of clinical symptoms ranging between sore throat, dry cough, fever, myalgias, and gastrointestinal symptoms such as nausea, anorexia, and diarrhea. Some infected patients also experience a temporary loss of smell and taste.
As the infection progresses to its second or third week, patients show signs of breathing difficulty, a range of hematological signs including lymphopenia and neutrophilia, and coagulation abnormalities such as pulmonary embolism, blood thickening, and rarely, some neurological symptoms.
Patients with severe COVID-19 can develop a condition known as a “cytokine storm,” in which cytokines, released by monocytes, lymphocytes, and alveolar macrophages that come in contact with the virus, give rise to destructive inflammatory responses.
Damage to the liver and a virus-induced increase in D‐dimers along with the cytokine storm alters the blood coagulation factors and causes DIC. Patients with fatal infection exhibit acute respiratory distress syndrome, myocardial injury, stroke, and multi-organ function damage during the final stages of infection.
Anti‐inflammatory therapeutics reduce the impact of cytokine storm and minimize severe disease
Amidst this unprecedented pandemic situation caused by a novel virus, the literature on the SARS‐CoV‐2 virus is evolving rapidly on a daily basis. Using the current understanding, the researchers proposed a mechanism that possibly drives coagulopathy in COVID‐19 patients.
According to their findings, the virus enters and binds to its receptor, and then attaches itself to the upper respiratory tract and lung tissues. The inflammation and hypoxic conditions caused by the infection lead to the release of TF, IL6, and other chemokines, which attract more lymphocytes and neutrophils into the lungs. Reactive immune molecules leak into the circulation and activate platelets and thrombin, triggering the clotting process. This results in the induction of a microthrombosis process, which causes PE, DIC, and multi-organ failure due to oxygen‐ and nutrient‐starvation.
The researchers believe that symptomatic treatment with anti‐inflammatory therapeutics and close monitoring of platelet counts and D‐dimer and fibrinogen levels might help mitigate the effects of cytokine storm and diagnose PE early in COVID‐19 patients. Also, using antiviral candidates such as remdesivir and hydroxychloroquine that target virus entry and replication could help speed up recovery times and bring down death rates. The team hopes that future studies will help improve understanding of various pathways contributing to thrombus formation in SARS‐CoV‐2-infected patients.
“Future observations and experimental studies will play a vital role in portraying a clear picture in understanding the physiological, molecular, and cellular signaling pathways that contribute to SARS‐CoV‐2–related thrombus formation.”
Source
Pujhari, S., Paul, S., Ahluwalia, J. and Rasgon, J.L. (2020), Clotting disorder in severe acute respiratory syndrome coronavirus 2. Rev Med Virol. doi:10.1002/rmv.2177, https://onlinelibrary.wiley.com/doi/10.1002/rmv.2177