Children with coronavirus disease 2019 (COVID-19) sometimes develop the Multisystem Inflammatory Syndrome in Children (MIS-C), which is associated with high morbidity. The underlying T-cell associated mechanism is described by researchers in a new study published on the preprint server bioRxiv* in November 2020, as a response to superantigen activation.
Toxic shock syndrome (TSS) and MIS-C share many similarities, with extensive and unregulated inflammation being characteristic of both. This study examined the occurrence of hyperinflammation in multiple body systems in children with COVID-19. These children experienced abdominal pain, diarrhea and rashes or had a weakening of the heart, sometimes involving cardiogenic shock. Strangely, these were not associated with severe respiratory symptoms. This suggests that MIS-C is due to extrapulmonary SARS-CoV-2 infection or a post-infectious inflammatory response.
TSS is also similar and is the result of superantigen stimulation, such as by Staphylococcal enterotoxin B (SEB). Superantigens are bacterial molecules that are very powerful at binding T cell receptors (TCR) and MHC class II molecules.
TCR V-beta skewing
The former binding engages specific beta-chains of the TCRs, involving their variable domains without having to rely on the complementary-determining region-3 (CDR3). This allows them to directly and nonspecifically activate the T cells and induce their proliferation, as well as elicit the unregulated hyperinflammatory cytokine-driven response (called a cytokine storm).
Specific superantigens bind to different TCR V-beta chains, and therefore T cells with these specific V-beta chains which bind to a diverse range of antigens will be overexpressed in these patients.
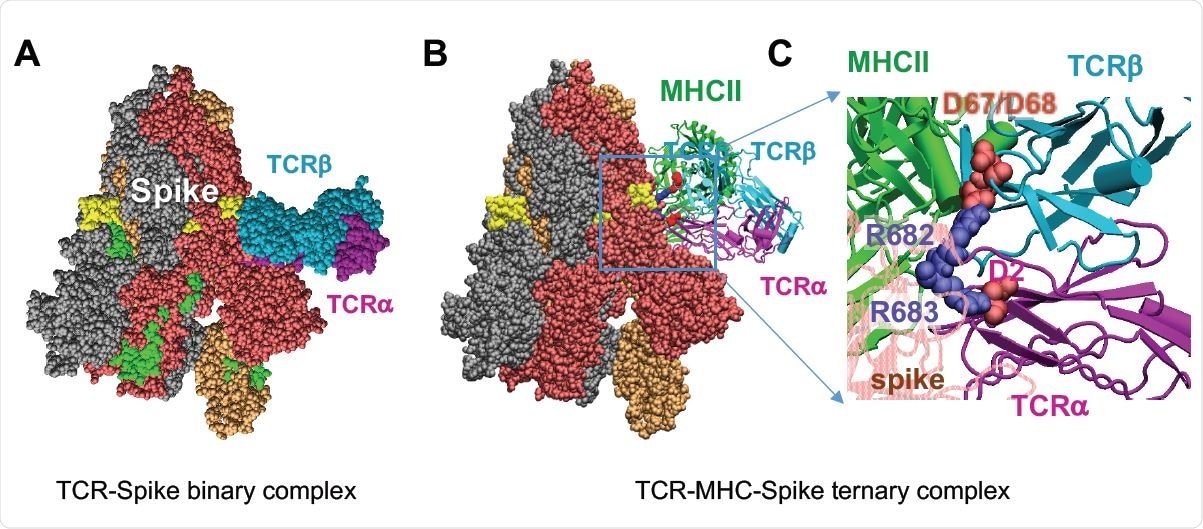
Complex Formation Between SARS-CoV-2 spike, TCR Containing Vβ11-2, and MHCII, and Comparative Analysis of TCR Vβ Sequences Homologous to Vβ11-2. (A) Binding of TCR (with Vβ chain sequentially identical to that of TRBV11-2 gene product) to the SAg-like region of SARS-CoV-2 spike. The TCR α- and β-chains are shown in magenta and cyan, respectively. The β-chain tightly binds the SAg-like region (E661 to R685; colored yellow). The spike subunits are colored dark red, beige, and gray; and the neurotoxin motif (299-356), green. (B-C) Ternary complex between spike, the same TCR and MHCII (green and the close-up view of the interfacial interactions between two basic residues, R682 and R683, on the SAg-like region of spike and the acidic residues (D67 and D68) of the TCR Vβ (D67 and D68) and TCRα.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Spike superantigen-like motif
The current study is based on the presence of a superantigen-like motif in the SARS-CoV-2 spike antigen. This binds to TCRs with high affinity as well as to MHC class II, forming a three-way complex. This motif closely resembles a staphylococcal superantigen in terms of sequence and structure. The TCR V-beta skewing seen in adults with severe hyperinflammatory COVID-19 phenotypes is similar to the type of immune response seen in superantigen-induced responses.
TCR beta variable gene expansion
The researchers explored the TCR range of expression in MIS-C with reference to V-beta skewing and signs of superantigen activation. They found that the TCR beta variable gene 11-2 (TRBV11-2) was greatly expanded. In mild MIS-C, there was a wider range of TCRs, just as in mild adult COVID-19, compared with severe MIS-C or COVID-19.
MIS-C patients showed an expansion of TRBV genes compared to patients with fever but without a SARS-CoV-2 infection. In severe disease, specific TRBV genes were exclusively over-represented, namely, TRBV11-2, TRBV24-1, and TRBV11-3 compared to either control with only fever or mild MIS-C patients.
The latter showed an expansion of TRBV-28 alone. Again, TRBV11-2 usage was correlated with the expression of inflammatory cytokines such as TNF-α, IFN-γ, IL-6 and IL-10 and with severe pediatric disease. This pattern was found to hold good irrespective of age in the severe MIS-C group.
Moreover, TRBV11-2 expansion correlated with PCR positivity rather than positive serology results. Over half the patients with this pattern were PCR positive, compared to zero patients without TRBV11-2 expansion. This data indicates an “association of TRBV11-2 expansion with active SARS-CoV-2 infection.”
Junctional diversity with TRBV11-2 expansion
Since CDR3 is not involved in superantigen interactions, the researchers expected a high degree of junctional diversity at the V(D)J junction in TCR with TRBV11-2 usage in severe MIS-C. This was confirmed by the finding that the CDR3/J genes in this subset of patients showed zero overlap, indicating high diversity. This again agrees with the canonical expansion profile observed with superantigen-induced activation.
TRAV8-4 was the most expanded gene among TRAVs in severe MIS-C patients, but no TRAV skewing was observed among mild MIS-C patients.
TRBV24-1 was overrepresented in adult patients with severe COVID-19, but less so in severe MIS-C. The single patient who had a robust expansion of the latter was a 15-year old.
Cytokine storm
TRBV11-2 expansion was correlated with the severity of MIS-C. Moreover, the serum cytokine levels in these patients agreed with those found in patients suffering from hyperimmune superantigen-triggered responses.
Severe MIS-C is associated with a cytokine storm, which can cause autoimmune antibodies to be formed against endothelial, immune cells and myocardial cells among others, indicating a loss of B cell tolerance of self-antigens. Superantigens also affect T cell-B cell antigens via their binding to B cell MHC class II, promoting their differentiation into immunoglobulin secreting cells and the activation of both polyclonal and monoclonal B cells.
TRBV11-2 binds to the spike antigen
The researchers also carried out modeling studies which indicate a CDR3-independent engagement of the TRBV11-2 with a polybasic insert P681RRAR, within the superantigen-like motif found on the SARS-CoV-2 spike protein.
Implications
The study concludes, “These data suggest that SARS-CoV-2 spike may act as a superantigen to trigger the development of MIS-C as well as cytokine storm in adult COVID-19 patients, with important implications for the development of therapeutic approaches.” This could include anti-SEB antibodies – which are found in most people over 12, but at lower levels in those over the age of 70 – or drugs that prevent superantigen engagement by mimicking the peptide.
Further exploration will reveal the phenotypic and functional characterization of the T cells that use TRBV11-2 in these patients, shedding light on the mechanisms behind the disease manifestations.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.