Researchers in the United States have defined an antigenic “supersite” within the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that could have important implications for the design of vaccines to protect against coronavirus disease 2019 (COVID-19).
The team – from Columbia University and the National Institute of Allergy and Infectious Diseases in Maryland – determined cryogenic electron microscopy (cryo-EM) structures for seven potent NTD-directed neutralizing antibodies.
All seven of the antibodies were found to target one common site at the periphery of the spike protein, thereby defining an NTD-antigenic supersite.
Lawrence Shapiro and colleagues say it is possible that all potent neutralizing antibodies directed at the spike NTD might target this one supersite.
The findings have important implications for vaccine design, which could start to include the NTD in formulations.
A pre-print version of the research paper is available on the bioRxiv* server while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Identifying viral sites vulnerable to neutralization
Since the first cases of SARS-CoV-2 were identified in Wuhan, China, late last year (2020), unprecedented efforts have been made worldwide to develop prophylactic and therapeutic approaches to protect against COVID-19.
One such approach is the identification and analysis of SARS-CoV-2 neutralizing antibodies to establish sites within the virus that are vulnerable to neutralization.
The primary target for neutralizing antibodies is the viral spike protein. This structure binds to the angiotensin-converting enzyme 2 (ACE2) receptor on host cells and mediates the fusion of the virus to the cell membrane.
The spike protein comprises two subunits. Subunit 1 (S1) contains the receptor-binding domain (RBD), the NTD and several other subdomains, while subunit 2 (S2) mediates virus-cell membrane fusion.
Most of the SARS-CoV-2 neutralizing antibodies identified to date target the RBD. Studies have revealed neutralizing antibodies that recognize the RBD at multiple distinct sites. They have also revealed classes of RBD-directed antibodies that appear to be elicited at a high rate, both in human and in animal models.
“While RBD-directed antibodies have been extensively studied, far less is known about NTD-directed antibodies,” says Shapiro and colleagues.
However, one study conducting EM analyses of antibodies from convalescent donors identified multiple NTD-neutralizing antibodies with potencies rivaling those of the best RBD-directed neutralizing antibodies, adds the team.
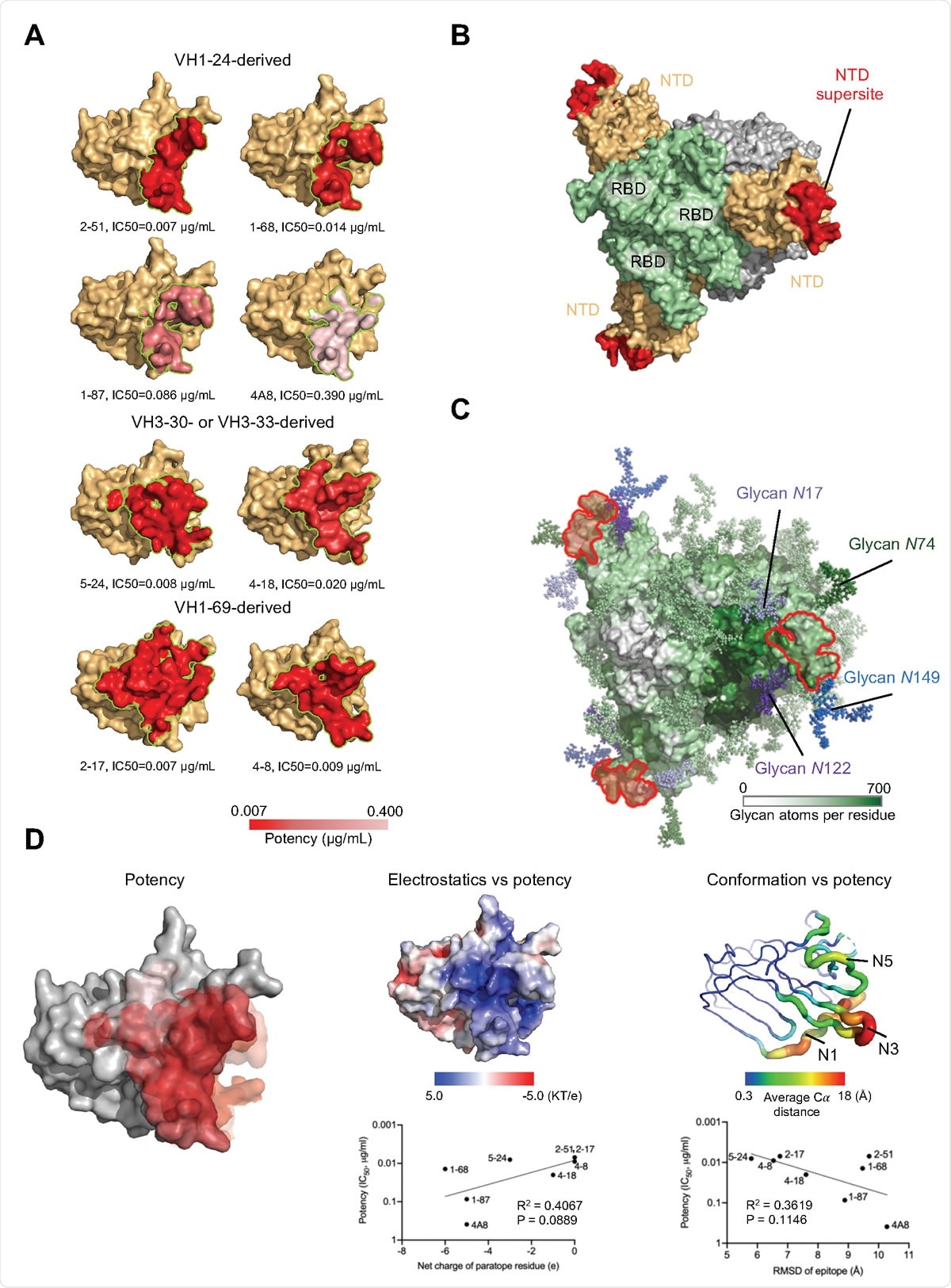
A structurally plastic antigenic supersite in the distal-loop region of NTD revealed by comparison of antibodies derived from the four multi-donor classes. (A) Epitopes of NTD-targeting antibodies colored by potency. (B) The supersite of vulnerability on NTD. (C) Glycan coverage of the spike. The NTD supersite is surrounded by glycans at N17, N74, N122 and N149. (D) NTD structural properties and antibody potency. Epitope surfaces of different antibodies were overlaid onto NTD with shades of red representing potency (left). Trending correlations were identified between antibody potency and epitope electrostatics (middle) and conformational change (right).
What did the current study involve?
Shapiro and colleagues have described cryo-EM and crystal structures for seven potent neutralizing antibodies in complex with either the spike protein or isolated NTD.
The team analyzed the genetic basis of recognition for the antibodies and classified them based on their genetics and modes of recognition. Several classes of antibodies were determined, at least one of which was seen in numerous convalescent donors.
Remarkably, analysis of the structures revealed that all seven antibodies targeted a single site on the NTD. This site was a large glycan-free surface located at the periphery of the spike protein and facing away from the viral membrane, defining an NTD-antigenic supersite.
“While RBD shows many non-overlapping sites of vulnerability to antibody, NTD appears to contain only a single site of vulnerability to neutralization,” writes the team.
Shapiro and colleagues say this may be due to the high glycan-density on the NTD, with few surfaces that are glycan-free and recognizable by the immune system.
“We propose that all potently neutralizing NTD-directed SARS-CoV-2 neutralizing antibodies might target this site,” they say.
What are the implications of the study?
The researchers say the study has clearly identified the site of vulnerability on NTD that is most likely to be targeted by neutralizing antibodies and that there are many ways it can be incorporated into vaccine design. Formulations could include the NTD in addition to the RBD and the NTD supersite could be displayed on nanoparticle immunogens, for example.
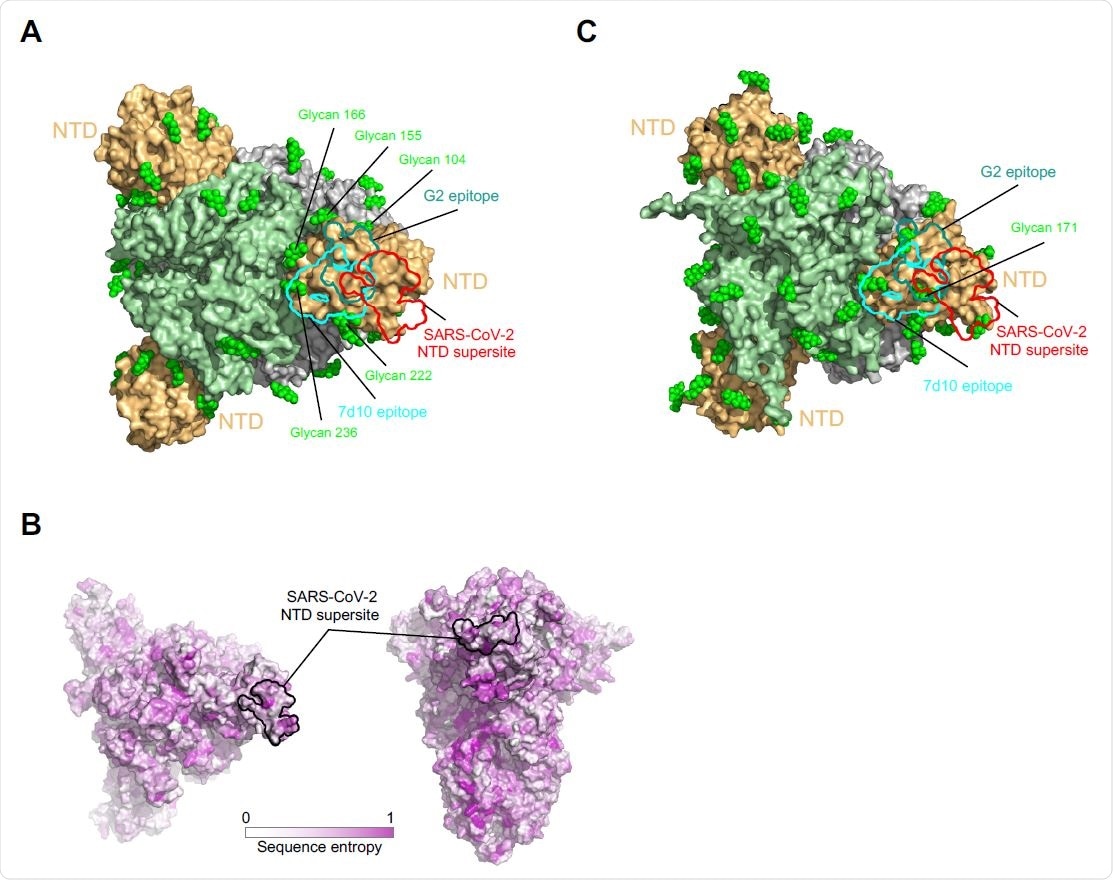
NTD supersite on MERS betacoronaviruses. (A) Epitopes of MERS NTD antibodies target a site closer to the trimer axis. Borders of epitopes of antibody G2 and 7d10 are colored teal and cyan, respectively. SARS-CoV-2 NTD supersite is shown as a red boundary line. Glycans are shown as green spheres. (B) Spike sequence entropy between betacoronaviruses. (C) NTD of HKU1 spike is substantially glycosylated.
In terms of therapeutic approaches, this supersite is remote from the sites targeted by RBD-directed antibodies, thereby providing the potential for complementary neutralization.
“The fact that all, or a great majority, of NTD-neutralizing antibodies, target a single site, however, suggests there may be little utility to utilizing combinations of NTD-directed neutralizing antibodies,” note the researchers.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources