In the year since the coronavirus disease 2019 (COVID-19) pandemic began, there has been a huge investment of research attention into the virus that causes it, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
As such, many experimental studies have taken place to help unravel the structure and biochemistry of the globally dominant variant of this virus, the D614G strain, as well as how this affects its function.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
A new preprint on the bioRxiv* server aims to develop a “unified view and a working model which is consistent with the diverse experimental data.” The scientists used a computational approach to integrate available data, and thus explore the molecular mechanisms of the D614 mutation. They applied atomistic modeling of the viral spike protein, conceiving of it as an allosteric regulatory mechanism.
The spike protein
The SARS-CoV-2 spike has a C-terminal and an N-terminal S1 and S2 subunit. The S1 mediates virus-receptor attachment, with an N-terminal domain (NTD) and the receptor-binding domain (RBD). The RBD is metastable, and continuously undergoes spontaneous conformational transformations, switching between the ‘down’ and ‘up’ positions. The spike can bind to the receptor only in the ‘up’ conformation.
The S2 mediates viral-host cell fusion. It is highly conserved, and contains numerous peptides. The S1 is a dynamic and protective shield of the fusion mechanism.
Upon viral binding to the host angiotensin-converting enzyme 2 (ACE2) receptor, the spike protein undergoes cleavage at the S1/S2 interface, and the two subunits dissociate. This triggers a series of conformational changes that mediate the fusion of viral and cell membranes.
Study aims
The study aimed at simulating the structure of both native and mutant SARS-CoV-2 spike proteins. For this purpose, the researchers made use of large-scale simulations, as well as analyses of the protein stability and dynamic fluctuation communication, and network-based community analysis.
D614G mutation subtly disrupts collective motions
Earlier, some scientists explained away the increased infectivity of the D614G mutant as being due to the altered conformational dynamics of the spike, with the open conformation being favored – the “openness” hypothesis. This would allow the spike RBD to make contact with the ACE2 host receptor.
This level of alteration was not observed in the current study. The use of CG-CABS simulations followed by atomistic reconstruction shows that the D614G mutation has very similar conformational dynamics to the wildtype in the closed and open states. The effect of the mutation is thus very delicate, comprising a large number of small and localized changes spread throughout the protein’s structure. These affect both inter-protomer and intra-protomer interactions, preferentially regulating some collective movements in the closed conformation.
A deeper look at how collective motions and rearrangements occur following the D614G mutation could throw more light into how this affects infectivity.
D614G modulates stability and allosteric communication propensity
The researchers also looked into the stability and the communication propensities of the amino acids in both the wildtype and mutant spike protein of the virus. They found that the D614 site, along with the Q613 site, anchor and incorporates major residues into regulatory or hinge centers that regulate global spike movements as well as allosteric changes in the structure of the spike in the closed and open state.
These hinge centers are the energetic hotspots, with both intra- and inter-protomer contacts occurring near these locations, mostly in the rigid S2 subunit. The D614G causes the largest shift in energy compared to other similar substitutions, with stabilization of both closed and open spike conformations.
The mutation stabilizes the spike trimer while reducing the premature shedding of the S1 domain – the “S1-shedding” hypothesis. This increases the number of functional spikes and the infectivity of the variant.
The D614G mutant, however, disrupts the hinge clusters and alters the functional movements in the trimer, which may increase the readiness of the spike protomers to shift to the open conformation. Thus, the stabilization effect of this mutation differs in the close and open states. This may help unify the “openness” and “S1-shedding” hypotheses accounting for the effect of this mutation.
Improved allosteric signaling in open state
The network community analysis of the SARS-CoV-2 spike proteins demonstrates the ability of this mutation to increase the number of stable communities in the open conformation, and of allosteric hubs for S1-S2 interdomain interactions.
Their findings show that the mutation is able to boost long-range signaling of the spike, by reorganizing allosteric interactions in the closed state. This increases the stability and the communication at the S1/S2 interface, with better allosteric signaling between the two domains in the open state.
At the same time, the mutation restricts S1 mobility, reducing its shedding, while enhancing the thermodynamic advantages of the open state. As a result, the RBD and NTD are potentially more exposed to the host receptor, supporting increased infectivity.
This supports the role of the mutation in favoring the open state by optimizing allosteric signaling in this state, and limiting S1 shedding.
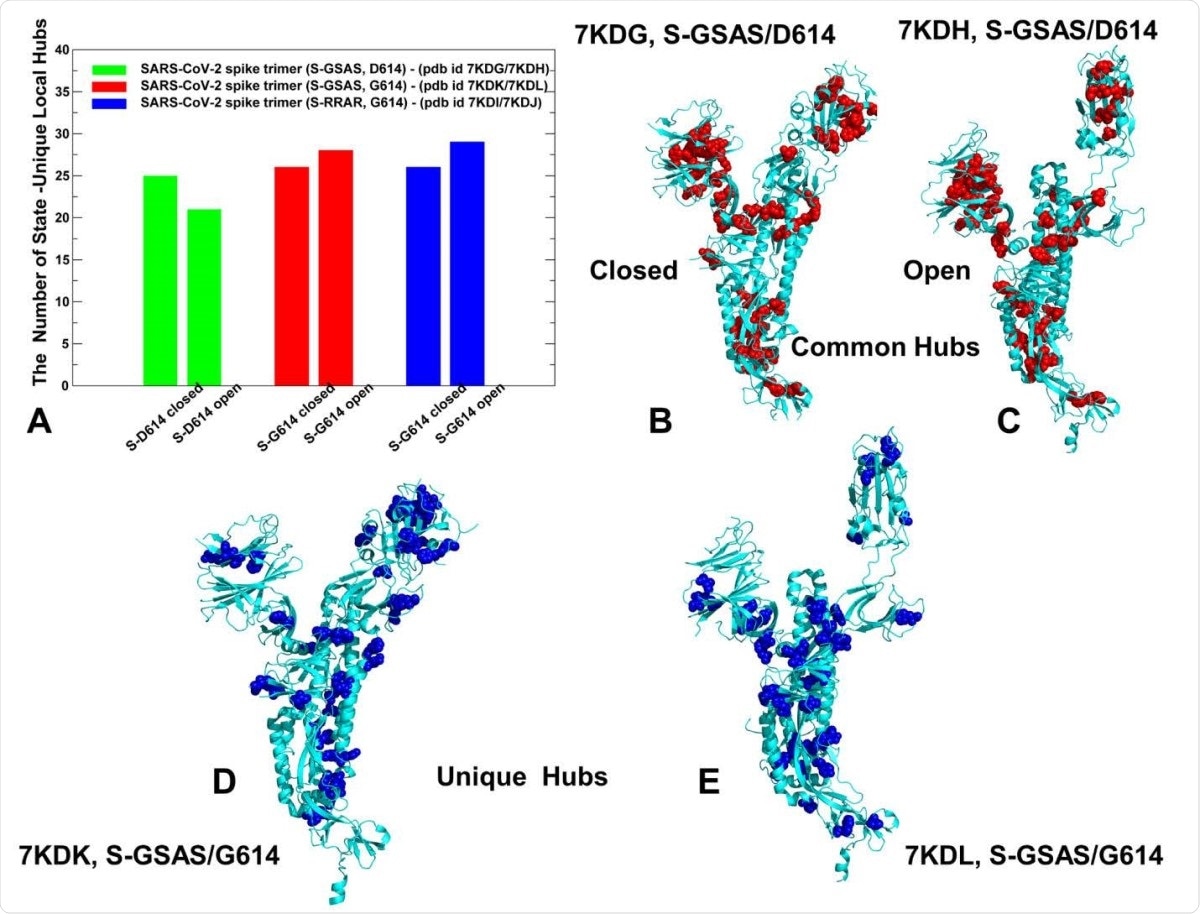
Network hub analysis of the SARS-CoV-2 S-D614 and S-D614G mutant structures. (A) The quantitative evaluation of the number of unique hubs in closed and open forms of SGSAS/ D614 (shown in green bars), closed and open forms of S-GSAS/G614 (red bars), and SRRAR/ G614 (blue bars). Structural mapping of common communities shared by closed and open states is projected onto a single protomer for the S-GSAS/D614 in the closed-all down state (B) and S-GSAS/D614 in the open state (C). The hubs are shown as red spheres. (D) Structural mapping of unique in the closed form of S-GSAS/G614 (pdb id 7KDK). (E) Structural mapping of unique hubs for the S-GSAS/G614 in the open state (pdb id 7KDL). The hubs are shown in blue spheres. The mapping is projected onto a single protomer shown in cyan ribbons.
What are the implications?
The study suggests the allosteric regulatory function of the D614G mutation, acting on both adjacent and remote sites. The results show that the D614 residue is one which commands the transitions of the receptor-binding domain (RBD) of the viral spike protein between the closed and open state. The D614G mutation can enhance the spike stability in both open and closed forms, but it allows the open form to be more thermodynamically favorable.
As a result, the researchers say, this mutation is associated with reduced shedding of the S1 domain of the spike protein. This in turn drives the higher infectivity of this strain.
The results reveal that the D614G mutation reduces S1 shedding, affecting the local interactions in the spike protein. The major effect continues to be via its allosteric effects on the stability of the spike, and communications within the protein residue networks.
This allosteric regulatory model could help explain how the D614G mutation brings about its effects. In the future, it may help explain how mutations enable immune evasion. Some recent studies show that the presence of D614G has a striking enhancing effect on the mutations in remote regions. These mutations in turn drive immune escape.
Considering functions of the SARS-CoV-2 pike proteins through the prism of an allosterically regulated machine may prove to be useful in uncovering functional mechanisms and rationalizing the growing body of diverse experimental data via allosteric models underpinning signaling events.”
Its broad impact could lead to an overall amplification of the effects of other mutations, explaining its rise to global dominance. Further studies on the mediating centers of the spike protein will help to understand the mechanisms of viral infectivity as well as to identify and develop suitable inhibitors, to effectively treat this infection.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources