Even as the coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to spread, new variants have emerged with increased infectiousness as well as virulence. This raises the threat of immune escape mediated by the array of mutations in these new variants.
Among these is the B.1.1.7 SARS-CoV-2 variant, with its characteristic N501Y mutation. A new preprint on the bioRxiv* server reports the impact of this mutation on CD4 T cell responses, including antigen presentation on cells expressing Major Histocompatibility Complex (MHC) class II molecules.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
The urgency of population immunity
The pandemic has already claimed well over 2.2 million lives little more than a year. This death toll has arisen from more than a hundred million reported infections worldwide.
Despite an intensive search for new and repurposed antivirals, success has been elusive to prevent and treat the infection. The major thrust continues to be on non-pharmaceutical interventions, including wearing masks, face and hand hygiene, and social distancing, with lockdowns being resorted to in scenarios where the case rates show an abrupt rise.
The definitive way out is the induction of herd immunity by both natural infection and vaccination. The immune response is signaled by seroconversion within seven days of infection. Durable immunoglobulin G (IgG) antibodies are detected in the patient, lasting for 2-3 months at steady levels before waning. Several studies have shown that immunity drops dramatically by six months post-infection.
More than 70% of convalescent COVID-19 patients have CD4 T cells reactive to SARS-CoV-2; their activity is directly proportional to specific IgG antibody titers. However, these cells are also reactive to the virus in 40-60% of unexposed individuals, which indicates that the seasonal “common cold” coronaviruses activate a subset of CD4 T cells that later recognize the common antigens on SARS-CoV-2.
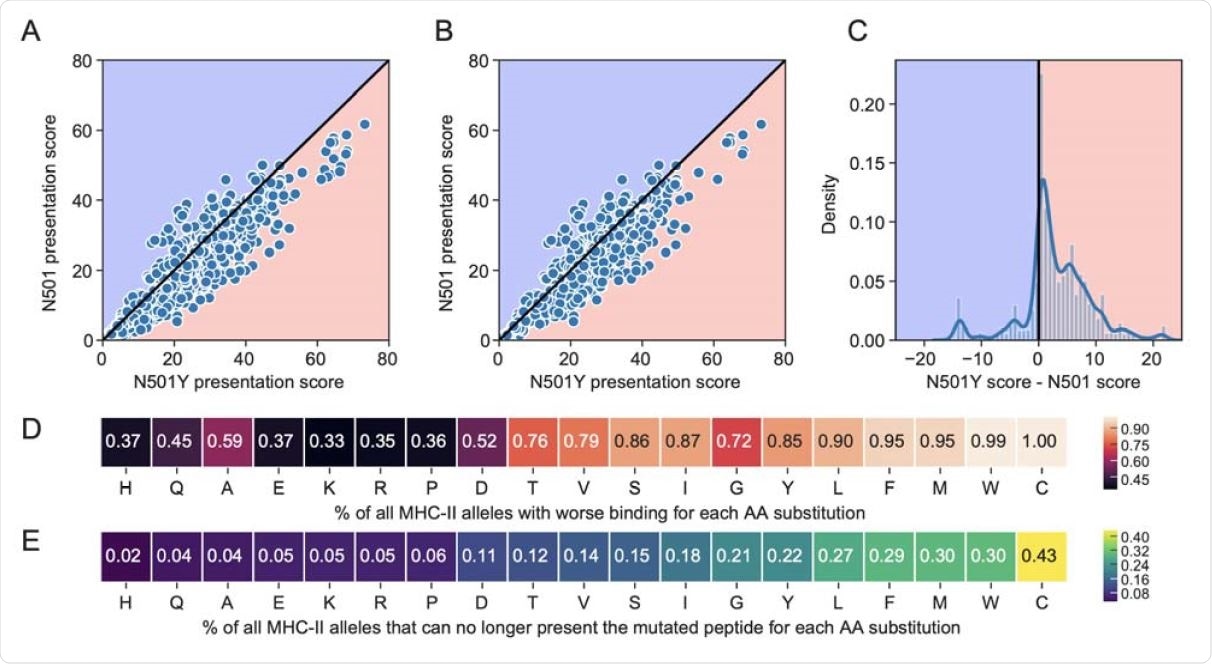
Comparison of percentile rank peptide-MHC binding scores for 15mer peptides spanning the N501 or N501Y position in the spike protein. The blue background indicates improved binding affinity (lower percentile rank) for a N501 peptide versus the corresponding N501Y mutated peptide, and a pink background indicates poorer binding affinity (higher percentile rank) for a N501 vs N501Y peptide. Scatterplots are shown for (A) all available 5620 MHC-II alleles, and (B) the 1911 most common MHC-II alleles. (C) Distribution of N501Y minus N501 MHC-II presentation scores across all MHC-II alleles. (D-E) Heatmaps for all possible single amino acid substitutions at position N501, showing the percent of MHC-II alleles that have (D) worsened binding, or (E) can no longer present the mutated peptide at a threshold of 20.
The rise of the UK variant
The so-called UK variant of the SARS-CoV-2 virus, B.1.1.7, was first reported following genome sequencing surveillance in December 2020. Since then, the sequence has been identified in more than 50 countries. This variant has been shown to be more infectious than the parent strain by 50%, causing it to form a significant and rising proportion of new cases in many regions.
In fact, the US Centers for Disease Prevention and Control (CDC) predict that the B.1.1.7 variant will dominate globally circulating strains by March 2021.
The N501Y mutation and infectivity
The characteristic N501Y mutation substitutes asparagine for tyrosine at this position in the receptor-binding domain (RBD) of the viral spike protein’s S1 subunit. The RBD is the region that makes direct contact with the host cell receptor, angiotensin-converting enzyme 2 (ACE2).
This mutation is common to many other variants, from South Africa, Brazil, Australia, Japan, Denmark, the Netherlands, and states in the USA such as Texas, Illinois, Louisiana, and Ohio. The presence of mutations at this residue seems to promote binding between the spike and ACE2, and the enhanced binding at this site perhaps explains the rapid rise to dominance of this strain.
N501Y and immune evasion
However, a bigger issue is on the minds of many; does this mutation also cause immune escape or contribute to it?
For one thing, the N501 residue is part of an RBD epitope that is a primary target of many neutralizing antibodies isolated from COVID-19 convalescents. As a result, the wild-type RBD could have elicited antibodies that fail to bind with equal effectiveness to the mutant RBD.
In fact, a mouse study showed increased infectivity and virulence with the mutant strain. The presence of some other specific mutations such as E484K may amplify the effect of the N501Y mutation on these characteristics of the virus.
MHC-II-mediated antigen presentation
Another explanation is that the mutation affects the adaptive immune response, which involves B and T cells. The MHC system coordinates the key molecule in this intricate dance of immune cells and antigens. This system comprises molecules on the cell surface that attaches only to antigen peptides of specific types, depending on the MHC class.
When the antigen forms a complex with the MHC, specific circulating T cells recognize it and expand their clone by proliferation. These T cells are capable of destroying the virus directly.
Also, the MHC-bound antigens activate CD4 T cells that in turn preferentially activate those B cells that are capable of specifically recognizing the antigen and generating a flood of anti-SARS-CoV-2 antibodies. These antibodies target the viral antigens and antigen fragments expressed upon antigen-presenting cells and on the infected cells themselves.
T-B cell coordination impaired
This orchestrated response of B and T cells is necessary to mount a robust immune response, perhaps by favoring the pairing of certain preferred pairs of B and T cell epitopes, or antibody binding sites.
The researchers in the current study set out to explore the effect of substitutions in the amino acids found around the B cell epitopes at the region where the RBD makes contact with the ACE2 receptor since these would necessarily have to bind MHC-II molecules as well, in order for T-B cooperation to occur.
They used computational modeling to predict the antigen presentation affinities for all the 15 peptides that may be found at the 501 position, in complex with MHC-II. Comparing the calculated presentation scores, they found that the N501Y mutant has less binding affinity for 85% of MHC-II alleles and 83% of the most common MHC-II alleles, compared to the parental peptide.
Not only so, but 22% of all alleles and of the common alleles are also unable to present the mutant peptide for further activation of T and B cells.
What are the implications?
These findings suggest, at least, the potential for lower MHC-II-mediated antigen presentation with the SARS-CoV-2 virus because of the lower binding affinity for MHC-II molecules at this site. The N501Y mutation could thus promote the more rapid spread of all such variants by its favorable effect on spike-ACE2 binding and its unfavorable impact on MHC-II binding for the majority of alleles.
The resulting weakening of T-B cell cooperation could well impair the production of anti-RBD neutralizing antibodies.
“The new virus variants with the critical N501Y mutation may pose challenges to the quality of the adaptive response required for protection.”
This is a grim possibility since it is relevant to natural immunity as well as that expected to be elicited by current vaccines. Future research must focus on demonstrating the ability to neutralize antibodies produced by natural infection or the vaccines in use to recognize and neutralize these new variants with the N501Y mutation.
The impact of this mutation on the binding strength and specificity of neutralizing antibodies must also be studied. And finally, if the CD4 T cells activated by contact with vaccine antigens containing the wild-type spike are biased to produce antibodies to the same antigenic sequence, including the original N501 residue, immune escape from the newer variants is likely.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.