Mucins and mucin-like molecules are highly glycosylated, high-molecular-weight cell surface proteins that possess a semi-rigid and highly extended extracellular domain.
PSGL-1 is a glycoprotein found on white blood cells and endothelial cells that binds to P-selectin (P stands for platelet), which is one of a family of selectins that includes E-selectin (endothelial) and L-selectin (leukocyte).
A new preprint posted to the bioRxiv* server demonstrates that similar mucins and mucin-like proteins cannot only suppress HIV-1 infectivity via blockage of viral attachment to the host cells but can block infection by multiple enveloped viruses. These include a number of related virion inhibitors belonging to the family of Surface-Hinged, Rigidly-Extended Killer (SHREK) proteins (PSGL-1, CD43, TIM-1, CD34, PODXL1, PODXL2, CD164, MUC1, MUC4, and TMEM123).
Mucin PGSL-1 deletion impairs antiviral response
Earlier, it was shown that when the abundantly glycosylated extracellular region of PGSL-1 was deleted, the protein lost its antiviral activity, indicating the essential nature of this region in blocking infectivity of the virus.
When the related mucins were tested individually for antiviral activity, they found that all of them blocked the infectivity of HIV virions. However, other mucins or mucin-like proteins with large extracellular repeat domains could not inhibit HIV infection.
This includes proteins with heavy glycosylation of the extracellular immunoglobulin domains, like CD2 and ICAM-1, or selectins with consensus repeats in their extracellular domain, or even integrin beta chain-2 (IGTB2) with its large extracellular domain containing integrin epidermal growth factor like repeat domains (I-EGF repeats).
Thus, the presence of transmembrane protein expression in cells within which viral replication is proceeding does not confer, unlike mucins and related proteins, the ability to block HIV infectivity. The latter group of proteins inhibits HIV in a dose-dependent manner, with CD34 being the most potent and MUCI the least. The 50% inhibitory concentration (IC50) was 2.3 ng and 216 ng, respectively.
The presence of potent and nonspecific antiviral activity, and common structural features, led to their being termed SHREK virion inactivators.
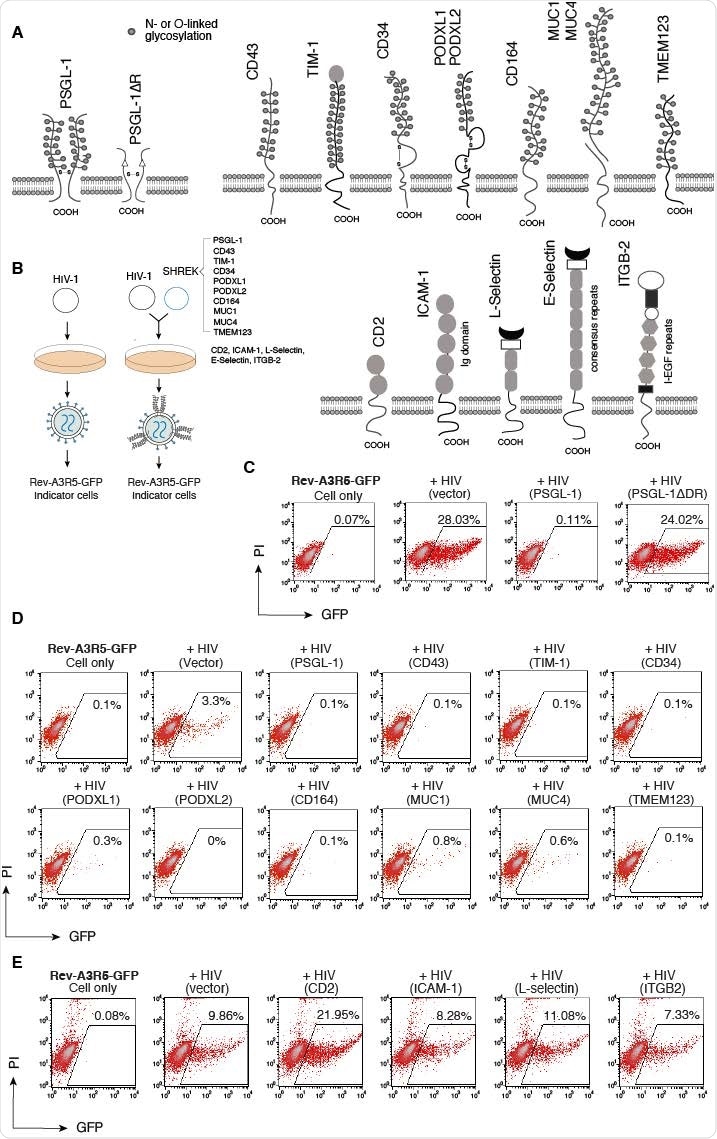
SHREK proteins inactivate HIV-1 virion infectivity. (A) Schematic of PSGL-1 and PSGL-1 ΔDR mutant, SHREK proteins, and other surface receptors used in this study. (B) Schematic of virion production in the presence of SHREK proteins in virus producer cells. (C) The requirement of the DR domain of PSGL-1 for blocking HIV-1 infectivity. HEK293T cells were cotransfected with HIV(NL4-3) DNA (1 μg) plus the vector expressing PSGL-1 or PSGL-1ΔDR (400ng). Virions were harvested at 48 hours post-transfection and normalized for p24, and viral infectivity was quantified by infecting Rev-A3R5-GFP indicator cells. HIV-1 replication was quantified by GFP expression at 72 hours post-infection. (D) SHREK proteins inactivate HIV-1 virion infectivity. HEK293T cells were cotransfected with HIV(NL4-3) DNA (1 μg ) plus each individual SHREK protein (500 ng). Virions were harvested at 48 hours and normalized for p24, and viral infectivity was quantified by infecting Rev-A3R5-GFP indicator cells. Shown are the percentages of GFP+ cells at 48 hours post-infection. (E) For comparison, cells were also similarly cotransfected with HIV-1(NL4-3) DNA plus an empty vector or the vector expressing CD2, ICAM-1, L-selectin, or ITGB2. Virions were harvested and quantified by infecting Rev-A3R5-GFP indicator cells.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Effects of SHREK on HIV release
When tested, SHREK proteins were found to not block virion release at low doses, below 100 ng, but were effective inhibitors of virion infectivity, with the IC50 being 10 ng or less. Thus, the latter is likely to be the primary effect of SHREK proteins on virus producer cells' virions, just like PSGL-1.
Incorporation of SHREK proteins into virions
The researchers found that PSGL-1 and other related mucins were incorporated into virions. This was confirmed by the selective binding of several anti-SHREK antibodies to virion particles produced in virus producer cells that expressed SHREK proteins. The exceptions were antibodies to TMEM123, MUC1 and MUC4.
This indicated that the mechanism of inhibition of HIV infectivity was via steric hindrance to the binding of virions to target cells. In the presence of almost all SHREKs, the incorporation of the HIV envelope protein was also blocked to variable but small degrees.
Blockade of virus-cell binding
Using virions produced from cells that expressed each SHREK protein, the researchers assayed viral attachment to target cells. They observed severely impaired attachment of virions from PSGL-1-expressing cells, as well as those expressing CD34 and PODXL2 – these three are the most potent SHREK virion inactivators.
However, the other SHREK proteins, except TMEM123, also inhibited virion attachment, but less powerfully. TMEM123 slightly increased virion attachment, perhaps by its binding to cell surface proteins, allowing the virion to attach but not to establish a productive infection. When such interactions were prevented using anti-TMEM123 antibodies, increased binding was no longer observed, but rather decreased attachment.
Broad-spectrum antiviral activity
The study demonstrated that SHREK proteins like PSGL-1 are indeed broad-spectrum host antiviral factors, acting to prevent infection by influenza A and SARS-CoV-2 virus-like particles (VLP). The most potent inhibition was with MUC1, PODXL1, and MUC4, for the influenza virus.
This indicates that each of the SHREKs has distinct antiviral potency against different viruses, determined by its location on the cell membrane, the site of viral budding, and viral antagonisms.
An inhibition assay was also performed using novel hybrid alphavirus-SARS-CoV-2 particles and the individual SHREKs. This particle contains only the structural SARS-CoV-2 proteins, with the RNA genome of the alphavirus. This hybrid's infection was successfully neutralized by many SHREKs, including PSGL-1, CD164, TIM-1, MUC1, and MUC4, but not by the potent HIV inhibitors CD43, CD34 PODXL1, PODXL2, and TMEM123.
What are the implications?
The study identifies a protein family with a similar structure and broad antiviral activity, called SHREKs by the researchers. They confirmed earlier findings that in the presence of PSGL-1 within host cells, the virion's infectivity was affected.
Other dissimilar proteins such as E-selectin may also turn out to be part of the SHREK family because this group of proteins has three mechanisms of action: inhibition of virion release, preventing the incorporation of viral attachment proteins such as HIV-1 gp160 into the virions, and the incorporation of SHREKs into the virions, thus preventing the attachment of the newly formed virions into target cells by steric interference.
Based on earlier research, they hypothesize that HIV and other viruses could also adapt to such SHREK-mediated inhibition by newly developed antagonistic mechanisms. One instance is the use of accessory proteins Vpu and Nef to break down PSGL-1 and reduce its expression on CD4 T cells. Other viral antagonisms may exist to individual SHREKs, though unknown as yet.
The high expression of SHREKs on stem and progenitor cells was a striking feature, especially of CD34, a common marker on such cells. This is the most potent anti-retrovirus SHREK observed in this study. Such proteins are probably part of the host's innate immune response to restrict retroviruses' replication in these essential cell subsets.
Earlier research has shown that cytokines like GM-CSF can induce the removal of CD34 from the cell surface in CD34+ multipotent hematopoietic progenitor cells (HPCs), and that this is required for permissive viral replication in such cells.
"Our identification of the SHREK family of proteins offers potential new antiviral therapeutics that may be developed through the induction and modulation of SHREK activities and the inhibition of viral antagonisms."

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources