Researchers in the United States and Chile have provided preliminary evidence that individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes coronavirus disease 2019 (COVID-19) – mount a robust neutralizing antibody response against the virus that can be significantly boosted by two doses of Sinovac Biotech’s CoronaVac vaccine or one dose of Pfizer-BioNTech’s BNT162b2 vaccine.
Immunization of infection-naïve individuals with two doses of CoronaVac or one dose of BNT162b2 also elicited similar levels of neutralizing antibodies to those observed in seropositive individuals 4.2 to 13.3 months post-infection.
“This preliminary evidence suggests that both seropositive and naïve individuals require two doses of CoronaVac to ensure the induction of robust nAb [neutralizing antibody] titers,” writes Rafael Medina from Pontificia Universidad Católica de Chile in Santiago and colleagues.
Importantly, the team says that three seropositive individuals who were obese failed to mount a booster response following vaccination – a finding that warrants further investigation.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
The durability of neutralizing antibody responses has become an important focus
During the ongoing COVID-19 pandemic, the durability of nAb responses to SARS-CoV-2 following infection or vaccination has become an important focus in efforts to determine correlates of protection against the disease.
Furthermore, the booster effect that vaccination might have on these responses remains undetermined.
Current evidence shows that while SARS-CoV-2-specific antibodies generated by infection decline over time, they are still detectable up to eight months after symptom-onset.
“However, additional longitudinal data are needed to characterize the medium- and long-term protective antibody dynamics, starting from the acute phase of disease among inpatients with mild and moderate-to-severe outcomes, and to determine their nAb memory response upon immunization with the different vaccines currently in use,” says Medina and the team.
What did the researchers do?
The researchers recruited 74 individuals (mean age 44 years [range 14 to 83]) with SARS-CoV-2 infection, as confirmed by reverse transcription-polymerase chain reaction (RT-PCR) testing.
Of these individuals, 37 were outpatients with mild disease and 37 were hospitalized patients with moderate-to-severe disease.
These patients were followed longitudinally to determine their nAb responses for up to one year, with samples collected between two and 414 days after symptom onset.
Irrespective of the level of disease severity, these SARS-CoV-2-seropositive individuals mounted a robust nAb response that peaked during the first month.
Although nAb titers declined over time, the responses were sustained in both outpatient and hospitalized patients for up to 12 months and none experienced re-infection.
The effects of vaccination in this patient group
Twenty-seven of the previously seropositive individuals were vaccinated during the study period with either the Coronavac vaccine developed by Sinovac Biotech or the BNT162b2 vaccine developed by Pfizer-BioNTech.
Outpatients and hospitalized individuals were vaccinated between 4.2 to 13.3 months (average 9.9 months) after symptom onset.
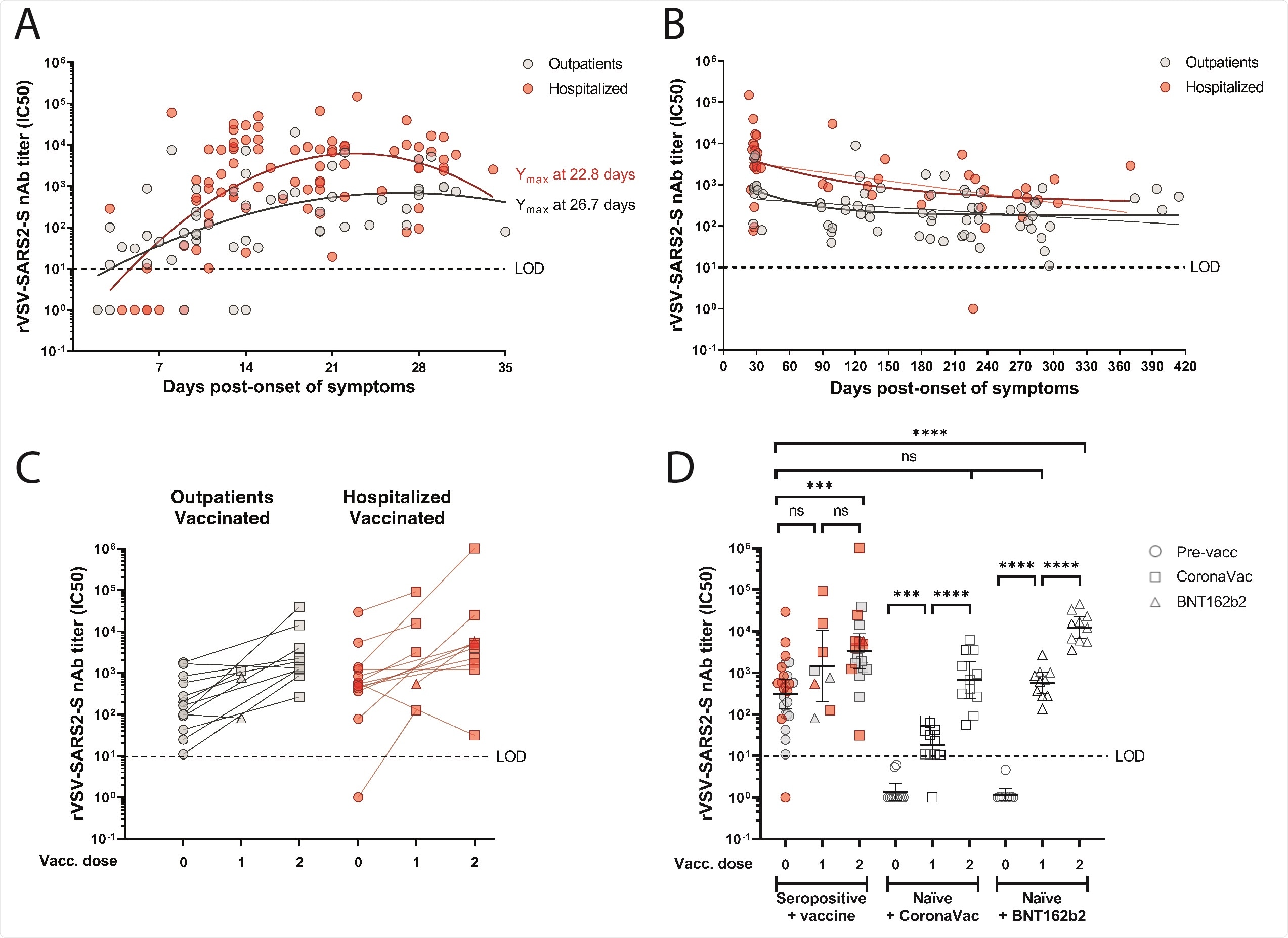
Neutralizing antibody responses to SARS-CoV-2 in seropositive and naïve individuals before and after CoronaVac or BNT162b2 vaccination. (Panels A to D) The half maximum inhibitory concentration (IC50) of sera was determined by microneutralization assay of recombinant vesicular stomatitis virus carrying SARS-CoV-2 spike protein (rVSV-SARS2-S). (Panel A) Neutralizing antibody (nAb) titers (IC50) from 15 outpatients (57 samples; grey circles) and 26 hospitalized (84 samples; red circles) at 2 to 36 days post-symptom onset. Second order polynomial (quadratic) curve fitting was used to establish the days at which peak titers occurred (Ymax). (Panel B) Longitudinal nAb titers from 36 outpatients (66 samples) and 31 hospitalized (44 samples) taken from day 27 (outpatients) or day 23 (hospitalized) until day 414 post-symptom onset. One-phase decay fit is indicated as a bold line, while continuous decay fit is shown with the thinner line in red and gray for the corresponding patient group. (Panel C) nAb titers from 13 outpatient (26 samples) or 14 hospitalized (28 samples) individuals immunized with one or two doses of CoronaVac (24 participants) or one or two doses of BNT162b2 (3 participants) vaccines. (Panel D) nAb titers from naïve individuals after the first and second dose of CoronaVac (11 participants) or BNT162b2 (10 participants) vaccines, compared to seropositive individuals who were not vaccinated (26 participants) or received one dose (8 samples) or two doses (20 samples) of the indicated vaccines. Geometric means with 95% confidence intervals are shown. Circles, non-vaccinated; squares, vaccinated with CoronaVac; triangles, vaccinated with BNT162b2. Dashed line indicates the limit of detection (LOD) of the microneutralization assay.
With the exception of four individuals, nAb responses were significantly boosted by two doses of the CoronaVac vaccine or one dose of the BNT162b2 vaccine, suggesting that immunization induced substantial B cell memory responses, says the team.
The nAb titers increased by an average of four-fold among outpatients and an average of three-fold among hospitalized patients after one dose. After the second dose, titers were increased by an average of 13-fold and 179-fold in these groups, respectively.
Of the four individuals for which no boosting effect was observed, one was an outpatient who had only been vaccinated two days previously and may therefore not have had sufficient time to mount a boosted response.
The remaining three were people with obesity, one of whom exhibited a significant decrease in nAb titer following two doses.
“Due to the high prevalence of obesity in severe COVID-19, further studies to monitor the induction of nAbs after vaccination in this population are required,” writes the team.
Comparing the boosting effect in seropositive and seronegative individuals
Next, the researchers assessed nAbs titers in healthy SARS-CoV-2 naïve (seronegative) individuals immunized with two doses of either CoronaVac or BNT162b2.
This revealed that two doses of the CoronaVac vaccine or one dose of the BNT162b2 vaccine elicited similar levels of nAbs in the seronegative individuals to those elicited in the seropositive individuals.
Among the previously infected individuals, vaccination with CoronaVac showed no significant differences in nAb titers after one dose or when comparing the first and second doses. Significantly increased nAb titers were only observed in this group following two doses of Coronavac.
What are the implications of the study?
The researchers say the findings suggest that natural SARS-CoV-2 infection induces long-lasting nAb responses that can be significantly boosted through vaccination. They also suggest that immunization of infection-naïve individuals with two doses of CoronaVac or one dose of BNT162b2 elicits similar titers of nAbs as in seropositive individuals 4.2 to 13.3 months post-infection.
In addition, “our preliminary evidence suggests that both seropositive and naïve individuals require two doses of CoronaVac to generate robust induction of nAb titers,” says Medina and colleagues.
“Further studies to determine the long-term duration of vaccine-induced nAbs against SARS-CoV-2 are warranted,” adds the team.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.